
|
March 2020,Volume 42, No.1
|
Update Article
|
How can imaging help in Parkinson’s disease diagnosis?Kin-lun Tsang 曾建倫 HK Pract 2020;42:10-14 SummaryParkinson’s disease from traditional teaching is a clinical diagnosis. Movement disorder experts can only achieve a diagnostic accuracy of 80% and this figure has not improved in the last 25 years. The difficulty is in the early stages when response to dopaminergic treatment is less defined and the hallmarks of alternative diagnoses such as atypical Parkinsonism may not have emerged. 1 With a better understanding of the pathogenesis of Parkinson’s disease, diagnostic investigations can now be used to target the integrity of the dopaminergic pathway and even to quantify it. 摘要傳統上柏金遜症依靠臨床診斷,近25年來診斷的準確率並沒有提升,運動障礙專家也只能達到80%。困難在於患者初期多巴胺藥物的效能可能不太明顯或非典型的症狀未完全出現。近來由於更加瞭解柏金遜症的發病機制, 令診斷檢測可以準確地評估多巴胺神經路徑的完整性,甚至可以加以量化。 IntroductionWhen a 60-year-old patient presents with a gradual onset of unilateral resting hand tremor, limb stiffness and slowness in walking; examination reveals mask facies, stooped posture with no arm swing, small-step gait and good postural stability, a diagnosis of idiopathic Parkinson’s disease (PD) is highly probable. However, if the patient’s tremor is predominant and the other signs are not very convincing, other differential diagnoses steps in. Essential tremor is common and can occasionally be debilitating but should not progress relentlessly. If slowness and stiffness are the main symptoms, other likely possibilities than PD are Vascular Parkinsonism, Drug-induced Parkinsonism, Multiple System Atrophy (MSA), Progressive Supranuclear Palsy (PSP) Atypical ParkinsonismIdiopathic Parkinson’s disease has a more confined degeneration, at least in the early stages, and response to symptomatic treatment is generally satisfactory. However, when degeneration is more extensive, additional features other than tremor, rigidity and slowness would be present and as such can be defined as specific entities (Table 1) . These are embraced as Atypical Parkinsonism which usually progress quicker and respond less well to treatment. 2 Making a correct diagnosis can avoid unnecessary trials of medication and allows the patient and his/her family to prepare plans for their long term care. 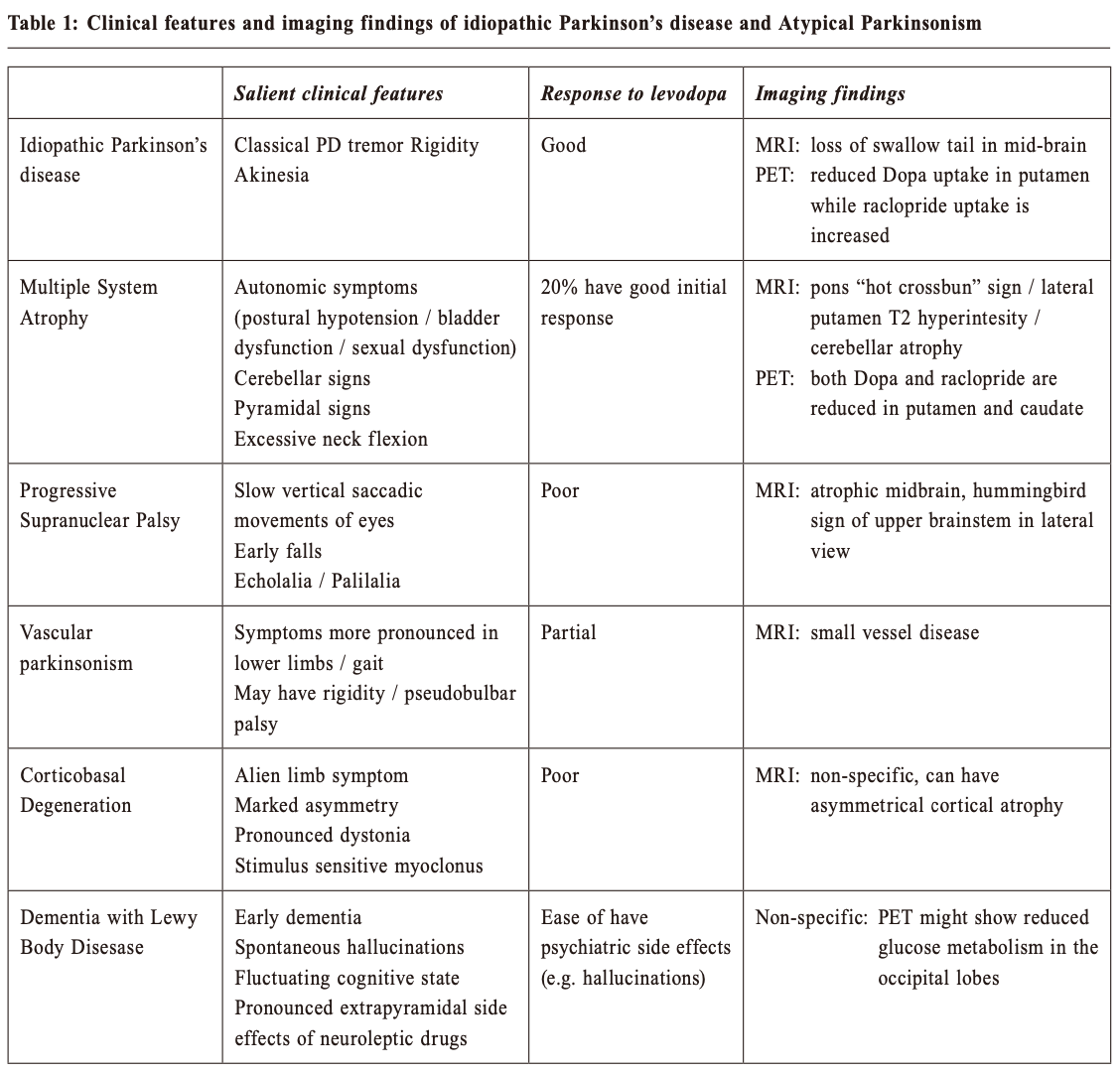
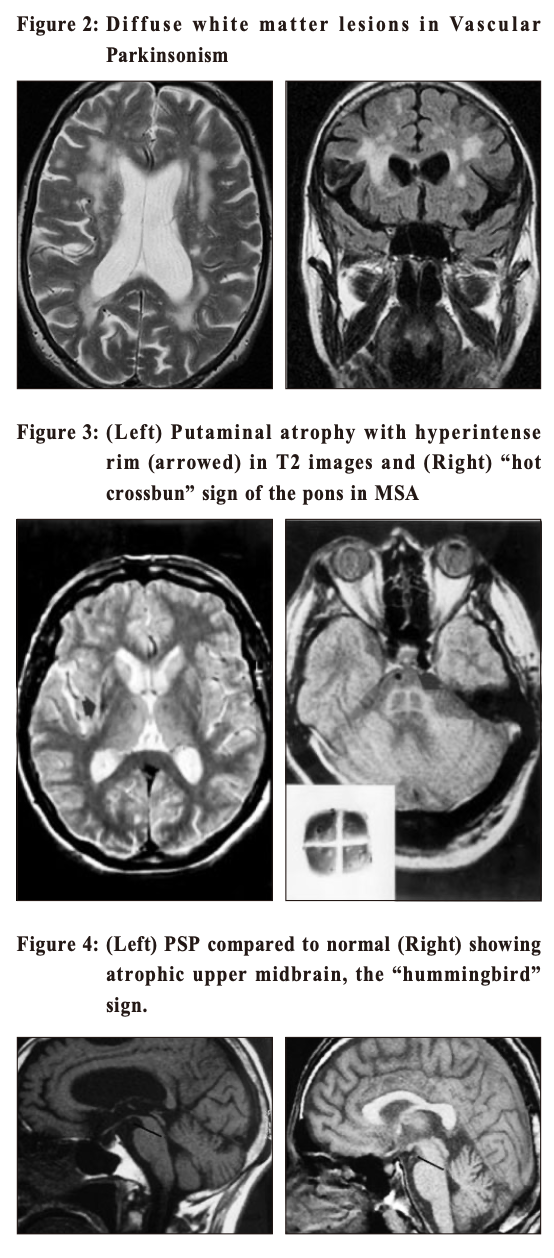
Multiple System Atrophy (MSA) shares with Parkinson’s disease in pathology of having abnormal alpha-synuclein proteins in the neurons. This is another neurodegenerative disease with a more rapid progression and a more extensive involvement. Patients can have cerebellar dysfunctions of gait, speech and limbs. Urogenital symptoms are common; with urinary incontinence, incomplete bladder emptying or erectile dysfunction in men. Autonomic involvements can lead to postural hypotension with fainting. Prevalence in the general population is around 5 per 100,000. Progressive Supranuclear Palsy(PSP) is even rarer. Its name points to the salient eye movement findings which one would find on examination of the patient. This is also a degenerative disorder and tau is the culprit pathological protein. Signs include Parkinsonism with prominent brainstem dysfunction of dysphagia, dysarthria and neck dystonia. Patients often suffer from frequent falls early in the disease process. Walking aid is often needed within 3 years of diagnosis and median survival is less than 10 years. Corticobasal Degeneration (CBD) is also a tauopathy with characteristic signs of profound dystonia and myoclonus, together with a sensation of having an “alien limb” as experienced by the patient. Vascular Parkinsonism (VP) is the typical example of “lower body parkinsonism”, with legs being affected much more than arms and hence gait impairment is substantial. 3 Patients are often older with a shorter history of illness onset and are more prone to postural instability, incontinence and dementia. White matter lesions (WMLs, as revealed on either CT or MRI) are the hallmark on imaging. Since vasculopathy is common, it is often encountered and should be kept in mind. If vascular risk factors can be promptly controlled, progression can be halted. Imaging study in patients with ParkinsonismWhen diagnosis of PD is doubtful, brain imaging study is often resorted to. But which one is the best? CT (Computerised Tomography) scanThis is easily available and cheap. It may help to rule out vascular disease and tumours as the cause of Parkinson-like symptoms. However, it does not have much of a positive value. MRI (Magnetic Resonance Imaging) scanMRI has a higher resolution and scans the brain in different sequences. Generally the public accepts this as the “gold standard” of imaging. However, it does not have much of a positive supportive value and only serves to rule out the rare possibilities of tumour or hydrocephalus as the cause of the patient’s “parkinsonism”. 4 Occasionally MRI of patients suffering from Idiopathic Parkinson’s disease (PD) can have the “swallow tail sign” in their substantia nigra, but this should only be regarded as a bonus rather than the norm (Figure 1). Similarly MRI will show small brain vessel disease in Vascular Parkinsonism (Figure 2). In MSA (Figure 3) and PSP (Figure 4), there might be tell-tale findings on their MRIs with funny names e.g. “hot cross-bun” and “humming bird” signs. 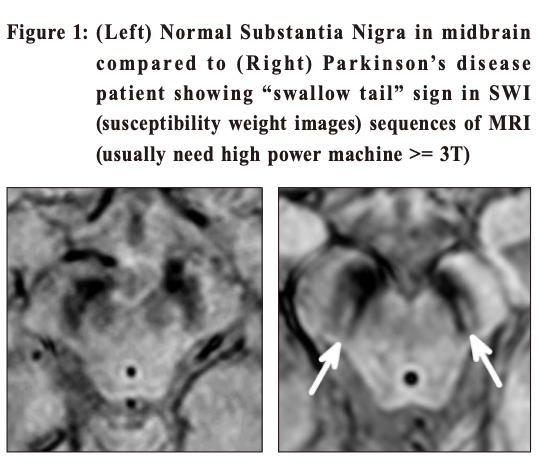
 Idiopathic Parkinson’s disease and ultrasoundTranscranial ultrasound has been studied as an imaging technique in the diagnosis of PD. 5 It is inexpensive. PD patients have an increased iron deposits in the substantia nigra and this will be hyperechogenic on ultrasound. However, this is highly operator dependent and sometimes ultrasound cannot penetrate through the skull bone window. Therefore it is still not commonly practised. Given that loss of dopamine producing cells is the hallmark in PD, confirmative imaging studies are functional scans to visualise the integrity of dopaminergic pathways of the brain. In Hong Kong, two options are available (both only available in the private sector) --- DaT and PET scans. DaT scanThe DaT scan (Dopamine Transporter) is a trade name and its proper name should be 123I-iof lupane SPECT imaging. Ioflupane has a high binding affinity for presynaptic dopamine transporters (DAT, a protein which retrieves dopamine from the synapses back into the neurons) in the brains of mammals, in particular the striatal (caudate, putamen and globus pallidus) region of the brain. 6The scan can quantify DAT which should be low in PD (Figure 5). It has been approved by the FDA in distinguishing essential tremor from Parkinsonian syndrome. It can also help to differentiate between Drug-induced or Vascular Parkinsonism (which should have normal or near-normal DAT). However, it cannot differentiate PD from Parkinsonian syndromes like MSA or PSP. 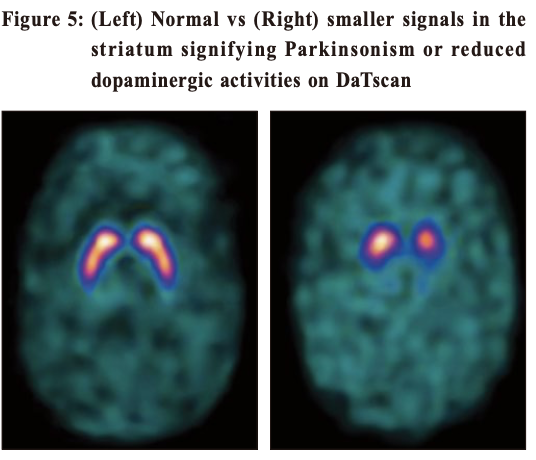
This scan involves receiving an injection of a radioactive drug (prepared in Europe and couriered to Hong Kong) that tags the dopamine transporters, and takes several hours to work. The scan itself then takes 30 to 45 minutes. The radioactive exposure, and risk association, is somewhat small. Potential side effects include headache, nausea, stomach upset, dry mouth and dizziness. PET (Positron Emission Tomography) scanPET scan for Parkinsonism is available in one private centre locally. It is expensive (nearly HK$30k) and is technically demanding because the isotope needs to be produced by an on-site cyclotron. It involves two scanning visits on two separate days. The protocol involves tracing the dopamine metabolism at the synapse (pre-synaptic), post-synaptic (dopaminergic, or specifically D2) receptor activities and brain glucose metabolism. 7 Thus, with so many variables being traced, differentiation of different neurodegenerative disorders, e.g. PD, MSA, PSP, Alzheimer’s disease, Corticobasal degeneration (Figure 6) is possible using this imaging investigation. 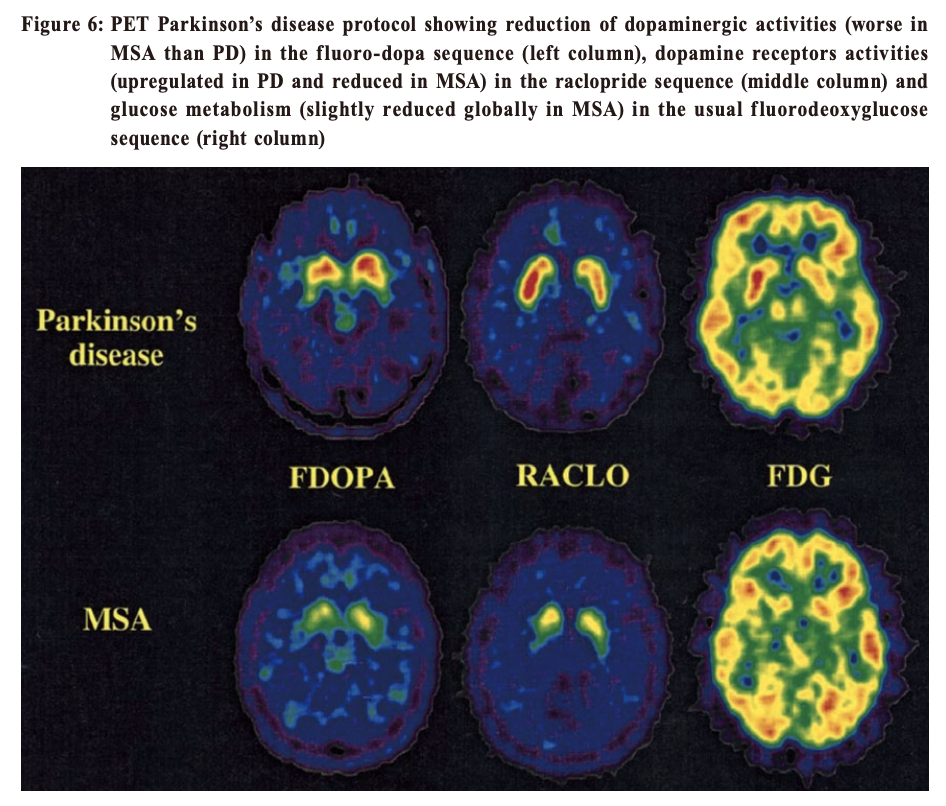
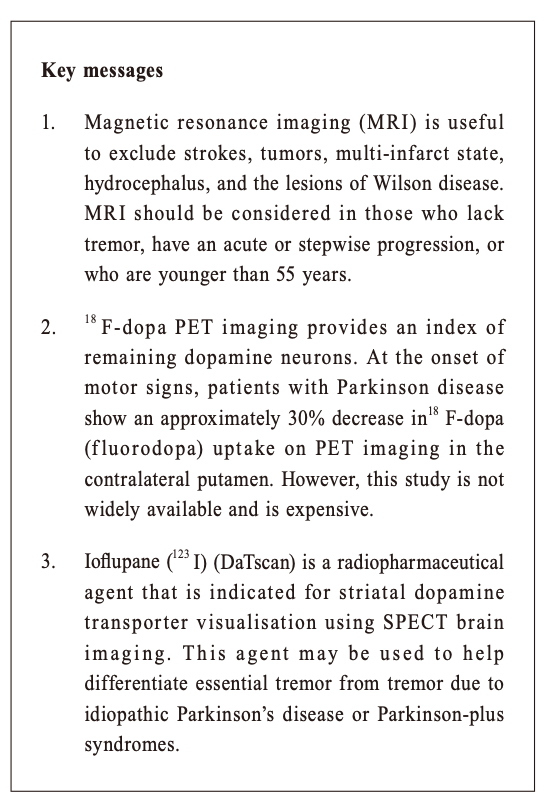
ConclusionDespite having all these sophisticated investigations, clinical examination and history taking is still of paramount importance in reaching the final diagnosis. Movement disorders specialists can have a diagnostic accuracy of 80%. If these specialists were wrong, the most likely underlying correct diagnoses are MSA or PSP. Tremor is the easiest sign to spot. Classical PD tremor should be of a “pill-rolling” type, with flexion of the thumb at 4-6Hz. Since the tremor is usually more pronounced when distracted, it should be observed when chatting with the patient or when they are mentally engaged with other tasks. Patients whose tremor is not typical should generally be considered for an MRI evaluation to exclude brain lesions such as stroke, tumour, hydrocephalus or demyelination. Patients younger than 40 years who present with Parkinsonian signs should have their serum ceruloplasmin checked to exclude Wilson disease. If the ceruloplasmin level is low, measurement of 24-hour urinary copper excretion and slit-lamp examination for Kayser-Fleischer rings must be performed. Suspicion of MSA can have autonomic testing or sphincter electromyography. Dopamine medications (dopamine agonists or levodopa) are often prescribed to patients with Parkinsonism symptoms, which is a reasonable option. Response to the medications can support the diagnosis of Parkinson’s disease. Over time, diagnostic accuracy improves as the progression of signs and symptoms and response to medications unfold. Imaging is to aid the diagnosis and repeat scanning is usually not necessary for monitoring, except for DaTscan which is quantitative and serial readings would show a decline in dopaminergic activities over time.
Kin-lun Tsang, , FHKAM (Med), FHKCP, FRCP (Edin), FRCP (Glasg)
Correspondence to:Dr Kin-lun Tsang, Room 1701, Crawford House, 70
Queen’s
Road, Central, Hong Kong SAR.
References:
|
|