
|
March 2020,Volume 42, No.1
|
Case Report
|
The first dermoscope-guided excisional biopsy for optimal clinical and cosmetic outcomes – a procedure performed in a primary care setting in Hong KongAntonio AT Chuh 許晏冬, Vijay Zawar, Regina Fölster-Holst HK Pract 2020;42:3-6 SummaryWe report here the first patient to have undergone dermoscope-guided excision biopsy, performed in Hong Kong with excellent optimal clinical and cosmetic outcomes. A facial dermal naevus was excised in the primary care setting. Complete excision and excellent cosmetic outcome was done. For this procedure, we rested the patient on a surgical couch, and secured a dermoscope head-down above the surgical field. Signals from the dermoscope were shown real-time on a monitor throughout the procedure. In this manner, three parameters can be adjusted precisely: (i) focus (allowing the superficial and deep structures to be in focus without changing the focal length), (ii) magnification (by altering the distance between the head of the scope and the surgical field), and (iii) the extent of epiluminescence (removing signals from the upper skin layers, thus allowing the deeper layers to be visible). However, the standards of the surgical equipments, hardwares, and softwares should be formalised. The formats of training are yet to be determined. Whether the incorporation of dermoscope-guided procedures in the primary care setting would lower the total medical expenditure in the community could also be explored in future studies. 摘要本文報告了首例皮膚鏡引導下的活檢切除術,患者取得了非常理想的臨床結果和美容效果。面部皮膚痣切除術是在基層醫療機構進行的,對皮膚痣做了徹底切除並取得了非常好的美容效果。 就此次手術而言,我們將患者置於手術臺上,並將皮膚鏡頭向下固定在手術區域上方。在整個手術過程中,皮膚鏡傳出的信號即時顯示在監視器上。 以此方式,可以對三個參數進行精准調節:(i)焦點(聚焦淺表和深層結構而無需改變焦距),(ii)放大率(通過改變鏡頭與手術區域之間的距離),(iii)皮表透光的範圍(去除上層皮膚的信號,使得深層皮膚可見)。 但尚應制定手術設備、硬體和軟體的相關標準,培訓模式亦尚待確定。在基層醫療機構引入皮膚鏡引導下的手術能否降低社區的醫療總費用,可在未來的研究中加以探討。 IntroductionDermoscopes are devices which could enhance the examination of lesions and abnormalities on the skin and skin appendages of patients. All dermoscopes can magnify skin lesions. Some allow the deeper layers of the skin and the lesions themselves to be visible. Most newer models achieve epiluminescence – the visualisation of deeper layers of the skin and lesions – via a mechanism known as cross-polarisation, utilising the physical properties of polarised light. The traditional indication for family physicians to apply dermoscopy was to detect skin cancers. If family physicians detect dermoscopic features of malignancies or suspected malignancies, they would refer these patients to dermatologists or other specialists. Over the past 15 years, the use of dermoscopy has been flourishing to detect and diagnose a wide range of skin problems. We have reported dermoscope-guided(DG) procedures, including DG-punch biopsy 1, DG-cautery 2, DG-laser ablation 2, and DG-suturing 3. We have also reported dermoscope-guided excisional biopsy (DGEB) of a potentially malignant skin mass subsequently found to be CD68+ and S100- juvenile xanthogranuloma on the thigh of a child. 4 We report here the first ever patient to have undergone DGEB with optimal clinical and cosmetic outcomes. To our best knowledge, this has not been reported by any other investigators. Case ReportOur setting consisted of two primary care surgeries attached to university teaching departments. Both surgeries were served by one family physician (AC) with a special interest in skin diseases. A male patient aged 49 consulted with a 15-year history of a non-painful mass on his face. He attended owing to a tinkling sensation in the lesion over the past two months. Our examination revealed a near-hemispherical hyper-pigmented lesion with comedone-like openings, in the middle of his left nasolabial fold (Figure 1). The longest diameter was 5 mm. Our provisional diagnosis was of a melanocytic naevus, being either compound or dermal. We explained to the patient the very low risk for dermal naevi to develop into malignancies, and possible modulations of intervention including excisional biopsy, carbon dioxide laser ablation, electrocautery, and cryosurgery with liquid nitrogen. We emphasised that complete excisional biopsy would be most favourable, as the entire lesion could be removed for histopathological investigations and to document complete or incomplete removal of the said lesion. We discussed with the patient the possibility of a referral to a plastic surgeon as well as giving him the option of DGEB. We requested that the patient re-attend again two weeks later for further discussion with regards his management decision. The patient opted for DGEB,and give his informed and written consent for the procedure. We rested the patient in a supine position on a surgical couch. We secured a dermoscope with the head down, and secured it via steel clamps directly above the surgical field. We then connected the dermoscope to a Personal Computer, which output signals to a monitor. We adjusted the focus of the dermoscope. We switched the magnification to 20X by altering the height of the dermoscope. The height of the dermoscope correlates inversely with its magnification power, i.e. the higher its position the lower is its magnification. Figure 2 depicts our setup for DGEB on another patient. After administration of perilesional anaesthetic agent, the investigator fixed his eyes mostly on the monitor during the procedure. We excised the lesion completely with a 2 mm margin, and attained haemostasis by fine pulses of carbon dioxide laser. Histopathology reviewed proliferation of naevus cells in the dermis with no junctional activity. The naeval cells were arranged in clusters and cords with maturation were well preserved. The naevus cells showed no cellular atypia, with melanin deposition in the more superficially located naevus cells. These features are compatible with the diagnosis of intradermal naevus. The patient attended for review one week post procedure. Evidence of early epithelisation was seen. Faint induration was noted around the wound. No complication were noted post-procedure. Figure 3 was taken two months after the procedure. Absolutely no scar was visible by this time. From the perspectives of a third person, the previous location of the removed naevus and whether the operation was performed on the right or left side of the face cannot be determined. 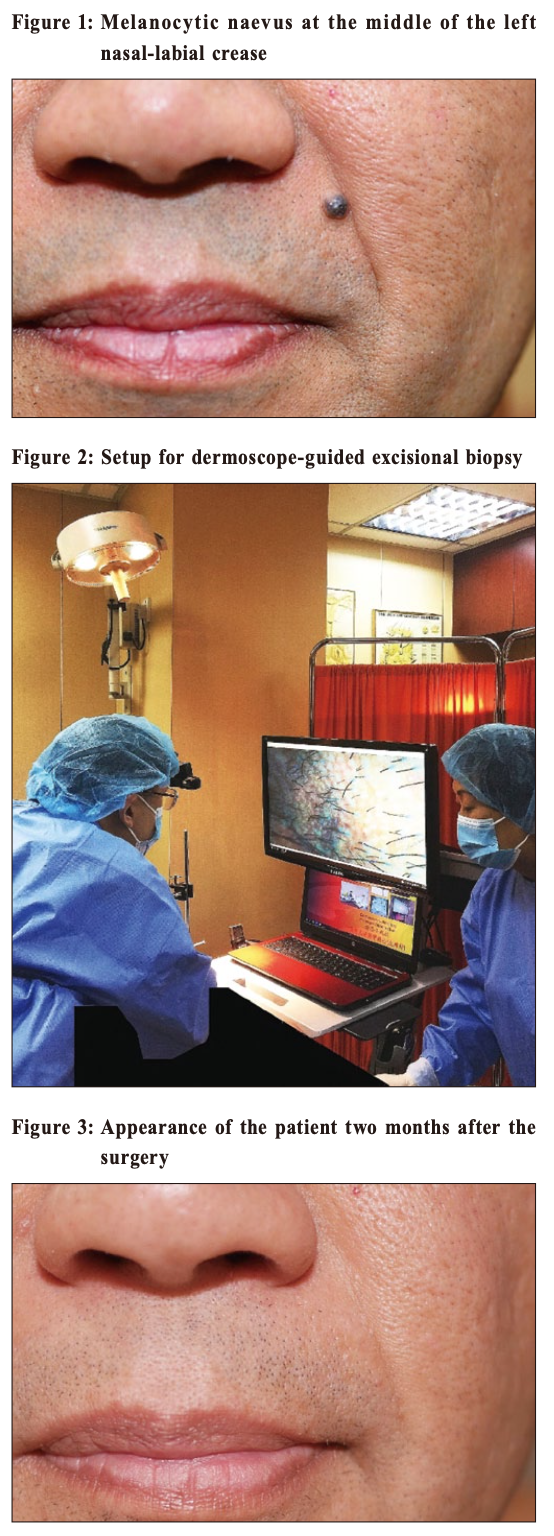
DiscussionThe terminology of dermoscope-guided excisional biopsy and other related operations should be clarified. Dermoscope-guided in this report refers to a dermoscope generating real-time images being displayed on a monitor during the operations.5 This real-time use of the dermoscope during surgical procedures differs from reports by other investigators, in which dermoscopy was applied to determine the margin of skin lesions or positions of incisions before operations 6, 7, or the application of dermoscopy to identify a site for incisional biopsy before operations, as reported by us 1, 5 and by other investigators. 8 Our procedure is also different from applying dermoscopes to assess the results after procedures or other treatment modalities. 9 In the present report, the clinical and dermoscopic diagnoses were identical – compound or intradermal naevus. As such, the risk of malignancy was virtually nil. With the lesion on the face and the risk of malignancy being very low, the cosmetic outcome would be of paramount pertinence. DCEB thus assisted us to operate with precision high enough to optimise both cosmetic outcome and complete removal of the lesion. However, for potentially malignant skin lesions, the top priority would be to ascertain the complete removal of the lesions. Cosmetic outcomes would necessarily be of a lower priority then. 5 The first advantage of DGEB over traditional operations might be magnification, which can be controlled by the family physician. The site of the lesion and intended incision would thus be precise. Such translates into lower rates of scar formation and incomplete excisions. The second advantage is epiluminescence, as covered above. Through cross-polarisation, the family physician could see beyond the upper layers of the surgical field. For small lesions, the entire mass down to the deepest parts and surfaces could be visible. The third is the high frame rate. Most digital dermoscopes output 30 frames per second. Such would allow the family physician to look at the screen in real-time smoothly. The fourth advantage for DGEB is that we could choose a particular scope for a particular procedure. While performing a procedure on a tiny lesion, for example, we used a dermoscope with the highest magnification. In another procedure for a thick lesion, we would go for the scope with the highest magnitude of epiluminescence. DGEB is particularly pertinent for family physicians. The incidences of skin malignancies in their settings are much lower than such for specialists, such as der matologists or cosmetic surgeons. It might thus not be cost-effective to train primary care clinicians in sophisticated procedures such as Mohs surgery. Moreover, the low rate of skin malignancies in primary care might be inadequate to re-validate their knowledge and skills regularly. 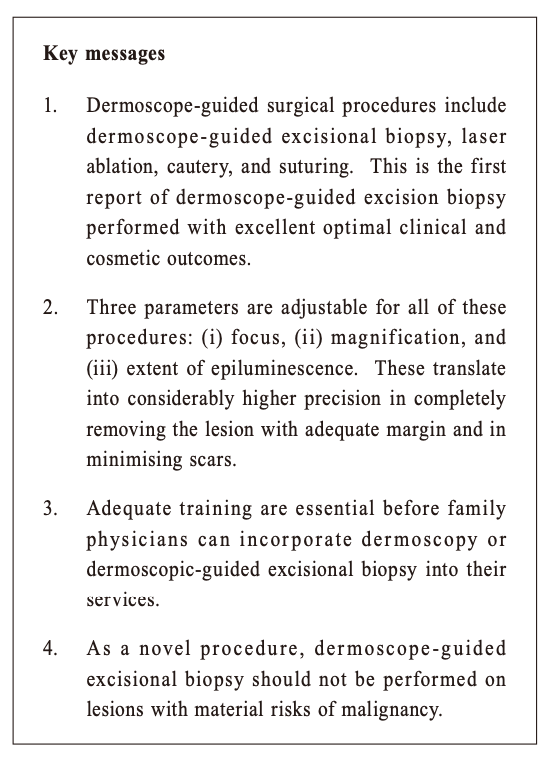
We have previously published a retrospective case-control study on 39 DG-surgical procedures performed by us and 39 control procedures on similar diseases and natures of procedures performed on 39 age (± five years) -and-sex pair-matched controls. We found that DG procedures were superior to conventional procedures with less incomplete excision of lesions or relapse of lesions (RR: 0.22, 95% CI: 0.05 - 0.95), and scarring for small lesions (RR: 0.30, 95% CI :0.13 - 0.67). 10 Further studies by us and other investigators might involve more DG-surgical procedures and control procedures. Blinding for the patients and the clinicians who have performed the procedures may not be feasible for studies on operative procedures. However, blinding of the assessors can be in place. More patient-assessed outcomes can also be incorporated. The results would then be more valid and reliable. Powers of the comparative results would also be elevated. However, as with other surgical procedures, suitable training has to be in place to apply dermoscopy and to conduct DG-surgical procedures. Being in the early stage of developing DGSP, we are not in a position to postulate the length and coverage of such training. The major disadvantage of DGEB is that it is novel. We are yet to explore long-term complications. We therefore recommend that DGEB should not be performed if the lesion could be malignant, if a provisional dermoscopic diagnosis was in the dark according to pattern analyses or other protocols, for facial lesions of some patients, and for lesions in the vicinities of important body parts such as the eyes, major blood vessels, or major nerves. We have thus reported the novel procedure of DGEB performed on a dermal melanocytic naevus on the face, with complete removal and excellent cosmetic outcome. We believe that DG-surgical procedures can be incorporated into the primary care setting, provided that the family physician is adequately trained and that high-quality instruments and softwares are in place. Whether the incorporation of DG-procedures in the primary care setting would lower the total medical expenditure in the community could also be explored in the future.
Antonio AT Chuh, MD, FRCP, FRCGP, FRCPCH
Vijay Zawar, MD, FRCPE
Regina Fölster-Holst,MD
Correspondence to: Dr Antonio AT Chuh, Shops 5 and 6, The Imperial
Terrac, 356
Queen’s Road West, G/F, Hong Kong SAR.
References:
|
|