
|
December 2018, Volume 40, No. 4
|
Update Article
|
Clinical review of the diagnosis and management of asthma in childrenWa-keung Chiu 趙華強 HK Pract 2018;40:125-134 Summary Asthma is the most common chronic disease in childhood with a prevalence of 10.2% of children in Hong Kong. There is no diagnostic test for asthma and its diagnosis remains a clinical judgement. Asthma is a disease characterised by chronic airway inflammation. It is defined by the history of episodic respiratory symptoms (wheezing, breathlessness, chest tightness and coughing) with variability and nocturnal worsening. Successful treatment of asthma involves 3 components: (1) controlling and avoiding asthma triggers, (2) monitoring asthmatic symptoms and lung function regularly, and (3) understanding how and when to use medications. Inhaled corticosteroid remains the mainstay of treatment of childhood asthma. However, detailed discussion with their parents is needed before its use. 摘要 哮喘是兒童期最常見的慢性病,香港兒童的患病率為10.2%。 診斷哮喘並沒有確診之檢測方法,主要靠臨床判斷。哮喘是 慢性氣道炎症的疾病,有間斷出現呼吸道症狀(喘鳴、氣 短、胸悶和咳嗽)的病史,會隨時間變化強度,且夜間加 重。哮喘的治療成功與否涉及三個要素:控制和避免哮喘的 引發因素;定期監測哮喘症狀和肺功能;瞭解如何及時用 藥。皮質類固醇吸入法仍是兒童期哮喘的主要治療方法,但 使用前應與患者進行詳細討論。 IntroductionAsthma is a disease with chronic airway inflammation. In asthmatic patient, this inflammation results in characteristic presentation of recurrent episodes of wheezing, breathlessness, chest tightness and coughing, which are more severe during night time and early morning. These episodes are featured by variable and often reversible expiratory airflow limitation. The bronchial hyper-responsiveness to stimuli, like allergen or irritant exposure, exercise, changes in weather, or viral respiratory infections, were developed secondary to the airway inflammation. The pathophysiology of asthma is defined by a variety of changes in the airway which included bronchial constriction, airway oedema, hyper-responsiveness and remodelling.1 EpidemiologyAsthma occurs in people of all ages worldwide. The most recent analyses of the Global Burden of Disease Study undertaken in 2008-2010, estimated that up to 334 million people were affected.2 Asthma usually develops in early childhood. Over 75% of children, who have asthmatic symptoms before age 7, will be symptom-free by age 16.2 According to the ISAAC III Study, the worldwide prevalence in the 6-7 and 13-14 years age groups were 11.7% and 14.1% respectively.3 The corresponding data in Hong Kong were 7.9% and 10.2% respectively and more than 330,000 people suffered from asthma locally.4 The burden of asthma is high, including absence from school and days lost from work. The mortality rate of asthma is reported to be 346,000 per year worldwide.5 Diagnosis6As there is no diagnostic test for asthma, the diagnosis remains a clinical one. One should recognise the characteristic pattern of respiratory symptoms (wheezing, breathlessness, chest tightness and coughing), physical signs (impaired growth parameters, chest deformity and wheeze) and test results (obstructive pattern in spirometry and increase exhaled nitric oxide). During the initial clinical assessment, one should look for the following features of asthma, including: 1. Recurrent episodes of symptoms with variabilityHistory of recurrent episodes of the above symptoms, especially if they are aggravated or triggered by exercise, inhaled alleyargen or viral infection, is highly suggestive of asthma. The diagnosis of asthma is suggested if the response to the bronchodilator treatment is positive. For patient with isolated cough, one should be cautious in making the diagnosis of asthma. 2. Physician diagnosed wheezeWheeze is medically defined as a continuous high-pitched sound with musical quality emitting from the chest during expiration.7 Parent reported wheeze is more heterogeneous. Noisy breathe, repeated or prolonged coughing with phlegm, respiratory distress may be the underlying reasons. One should be cautious in making the diagnosis of asthma. In fact, wheeze confirmed by a health care professional is more specific8 and it correlates with poorer lung function9. 3. Increase nocturnal symptomsThe asthmatic patients often suffer from night-time wheezing, breathlessness, chest tightness, coughing and post-tussive emesis that disturb their sleep. 4. Atopic historyA personal history or a family history of asthma, allergic rhinitis or eczema may increase the suspicion of asthma 5. Absence of features suggestive of alternative diagnosesOne should be alert if the patients have family history of bronchiectasis, signs and symptoms of abnormal cry or voice, swallowing difficulty, excessive vomiting, failure to thrive, inspiratory stridor, nasal polyps and persistent phlegmy cough symptoms since birth. Children >4 years of age and have all the above features should be given therapeutic trial of treatment e.g. 6-weeks of inhaled corticosteroids (ICSs), after detailed discussion with their parents. The patients’ progress should be assessed with Asthma Questionnaire (see Point 2 of Treatment) and/or lung function tests (FEV1 or serial peak flows if age appropriate) during follow-up. A good response to treatment is suggestive of asthma. Children ≤4 years of age or those not fulfilling the above features, having symptoms suggestive of other diagnoses, severe life-threatening attack or poor response to initial treatment should be referred to paediatric respirologist for further investigations. Further investigations including spirometry (with bronchodilator reversibility), challenge tests and/or measurement of fractional exhaled nitric oxide (FeNO) will be considered. TreatmentThere is no cure for asthma, but asthmatic symptoms can be controlled with effective treatment and management. For most of the asthmatic children, symptoms can be controlled with treatment and they can have normal growth and development, with normal level of daily activities, including sports. Successful treatment of asthma involves 3 components10:
Triggers are the factors that worsen asthmatic symptoms, and should be identified and avoided if possible. Common asthma triggers include:
The healthcare provider should work together with the parents to formulate a plan to avoid or limit the trigger as far as possible. Ask the parents to quit smoking. Remove furry pets and hairy dolls from home. Vacuum-clean the floor daily. Wash the sheets and other bedding in hot water at least once per week. Beware of household chemicals including soap, shampoo and detergent. Limit the use of pesticide sprays. Teach the patients to take precaution to exercise induced asthma by doing warm-up exercise and using bronchodilator before exercise. Refer those children with persistent problems to the paediatric respirologist. 2. Monitoring asthma symptoms and lung functionParent and/or child should keep an Asthma Diary to monitor the progress of the disease. It includes the frequency and severity of symptoms, the measurement of the peak expiratory flow rate (PEFR), and a standardised questionnaire like Asthma Control Test (ACT). ACT composes of 7 simple questions for children between 4 years old and 11 years old; and 5 questions for children aged 12 or above. It is useful to track asthmatic symptoms.11 Lung function assessmentLung function assessment by means of PEFR measurement or spirometry should be performed during the outpatient visits for children over the age of six years. It will guide the treatment decisions. 3. Classification of the severity of Asthma according to the clinical features before treatmentAsthma can be classified into intermittent and persistent asthma. Intermittent asthma is defined as having symptoms of asthma ≤1 per week which does not interfere with daily activities, nocturnal awakenings during the night due to asthma symptoms ≤2 per month, and lung function ≥ 80% predicted. Persistent asthma is defined as having symptoms regularly. There may be days when activities are limited due to asthmatic symptoms, and the child may be awakened from sleep. Lung function is usually normal between episodes, but becomes abnormal during an asthmatic attack. Persistent asthma can be mild, moderate, or severe in severity. Consultation with a paediatric respirologist is recommended for children who have moderate or severe persistent asthma, as well as those ages 0-4 years who have any form of persistent asthma. See: Table 112 
MedicationsA. Route of administration5Inhaled therapy is the mainstay of treatment for asthmatic children of all ages. The advantages include delivery of the drug to the site of action directly, hence a faster onset of action and a smaller required dosage; and minimises systemic absorption and its side effects. Short-acting beta-agonists (SABA) are generally targeted to more proximal airway which contain circumferential smooth muscle. On the other hand, the inhaled corticosteroids (ICSs) are targeted to more distal airway which account for most of the total airway surface area and have an increasing density of corticosteroid receptors.13 The choice of drug delivery device must be individualised. Various factors including the age of the patients, co-ordination, and inhalation technique must be considered. It is always not an easy task to use an inhalation device properly. The doctors or other health care professionals should regularly review the patients’ techniques. By using the device properly and adherence to the medication as prescribed, the asthmatic patients will have the best symptom control. Pressurised metered dose inhalers (pMDI), dry powder inhalers (DPI), and nebulizers, are the three principal types of inhalation drug delivery device available. Spacers with facemask or mouthpiece can be used as adjuncts to improve inhalation treatment. For children, pMDI is difficult to use properly as good coordination is needed. Otherwise the drug emitted will be deposited in the oropharynx. It should only be considered in children > 8 years of age. Spacers with facemask or mouthpiece were developed to overcome the difficulties of pMDI. With spacers, the patient can breathe tidally from a reservoir of drug. They are recommended for children <5 years of age. Delivery of drug by a mouthpiece is more efficient than a facemask and should be used as early as possible. Moreover, use of the spacer decreases oral and gastrointestinal absorption and hence reduce the potential oropharyngeal and systemic side effects. Therefore, it is the author’s practice to use a spacer if ICS is prescribed. DPI overcomes the coordination difficulties of pMDI and is more convenient to carry. It is recommended to use after age of 5.14 A nebulizer is a device to change liquid medication into a fine mist which can be inhaled through a mouthpiece or mask. There are two types of nebulizers: jet compressors and ultrasonic systems. The jet nebulizers use compressed air to generate small aerosol droplets while ultrasonic systems work by sound vibrations. They do not rely on patient cooperation or coordination to work. However, only less than 10% of the prescribed dose reaches the lung. They are recommended to use in children who cannot use a spacer.15 During asthmatic attacks, bronchodilator should be given via a spacer with a pMDI or by nebulizer with isolation precaution. In mild or moderate attacks, they are equally effective.16 Common inhaler devices for use by children, together with features of optimal inhalation technique are summarised in Table 2.5 B. Quick-relief medications for asthma1. Short-acting beta-agonists (SABA)SABAs are usually used clinically to treat acute asthma because of its effectiveness. Because of its direct action to the airway smooth muscle, the inhaled route allows a more rapid onset of action at a lower dosage with lesser side effects than other routes. Inhaled therapy can be used for prophylaxis of exercise induced asthma. Oral therapy is only used in young children who have difficulty in using inhaled treatment. Side effects are usually clinically insignificant except in high dose, these include muscle tremor, headache, palpiations, and agitation. 2. Short-acting anticholinergicsInhaled ipratropium bromide is clinically less effective in comparison with SABAs. It is usually used as an add-on treatment to SABAs and produce a better bronchodilation effect in acute severe asthma.17 Side effects are minimal and include mouth dryness and a bitter taste. C. Controller medications for asthmaChildren with persistent asthma need to take daily medication to achieve and maintain control of symptoms. The major medications are inhaled corticosteroids, leukotriene receptor antagonists, and long-acting beta2 agonists. The mechanism of action is to decrease the airway inflammation. 1. Inhaled Corticosteroids (ICS)The most effective anti-inflammatory medication in asthma is ICS. In mild and moderate asthmatic patients not receiving Oral Corticosteroids (OCS), ICSs with Fluticasone Propionate18 or Budesonide19 resulted in improvements in lung functions; symptom scores and reduction in bronchodilator use, in comparison with placebo. For those on OCS, ICS use resulted in significant reduction in the number of patients who were able to tail off the medication. Concerning the starting dose of ICS, doubling of ICS and then stepdown in comparison with a low dose did not show significant differences in lung function, symptoms, rescue medications or asthma control between the two treatment approaches.20 Symptom control and improvement in lung function occur rapidly after 1-2 weeks.21 Patients with well-controlled asthma who stop regular use of ICS have an increased risk of an asthma exacerbation compared with those who continue ICS.22 For infants and preschool children with recurrent wheezing/asthma of at least 6 months, a meta-analysis involving up to 3,592 subjects revealed that those received ICS had significantly less attacks than the placebo group (18.0% vs 32.1%). Moreover, treatment with ICS resulted in significantly less bronchodilator use, fewer withdrawals, and more clinical and functional improvement.23 However, treatment with ICS for children up to 2 years of age was not disease-modifying; symptoms almost always return when treatment discontinued.24 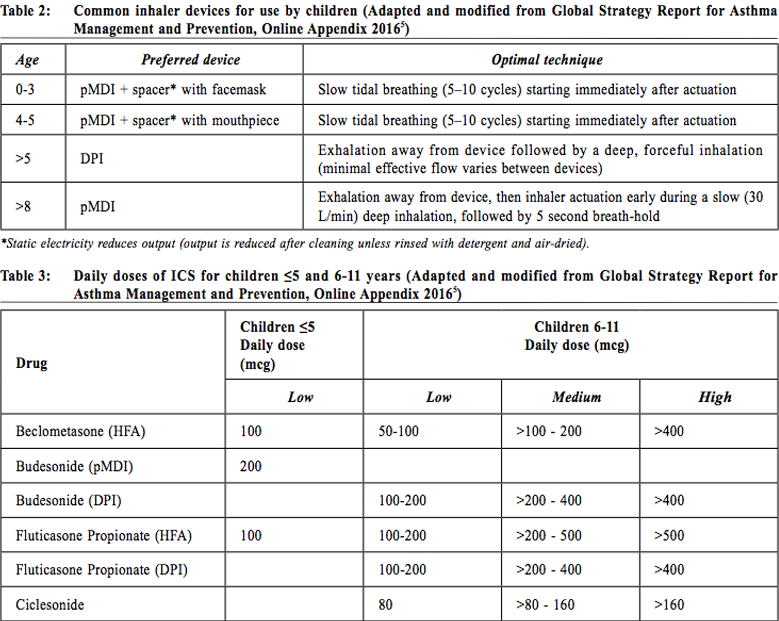
Intermittent Vs daily ICSAsthma guidelines recommend the use of daily ICS for prophylaxis against mild-to-moderate persistent asthma. However, there is always the problem of adherence to the treatment when symptom-free. Therefore, intermittent ICS in response to symptoms become an emerging strategy to deal with the problem. Additionally, this intermittent ICS strategy could potentially reduce costs and long term adverse events. A systematic review with meta-analysis in 2013 revealed no statistically significant difference in the rate of asthma exacerbations and pulmonary function between the two strategies. Daily ICS use has an advantage of 10% increase in asthma-free days and a trend towards decrease in rescue SABA use. Moreover, intermittent ICS use was associated with increasing exhaled nitric oxide and eosinophils in sputum. However, daily ICS use was associated with greater exposure to ICS and a small and non-significant decline in the short-term linear growth rate during treatment.25 For children and adult patients with mild persistent asthma and not taking daily ICS as prophylaxis, the Cochrane Review in 2015 showed that the use of intermittent ICS during attack reduced the use of oral corticosteroids (OCSs). Similar results were found in preschool children with wheeze, with an improvement of asthma symptoms and quality of life. Hospitalisation rates were similar among all age groups. There was no growth suppression or other adverse effects noted.26 Side effects of ICS are usually minimal, including oral candidiasis and hoarseness of voice. There are no increased risk of cataract, fracture and tuberculosis; as well as hyperactive behaviour and aggressiveness. For the endocrine effects, treatment with low dose ICS daily is not normally associated with any significant suppression of the hypothalamic- pituitary axis in children. Concerning the growth, regular ICS use in children with asthma at low or medium doses is associated with a decrease in linear growth velocity and height during a one-year treatment of 0.48 and 0.61 cm/year respectively. The growth suppression seems to be less pronounced after the first year. However, one should also aware that uncontrolled or severe asthma adversely affects growth and final adult height.27 2. Combination ICS/LABAs (long-acting-betaagonist)Long-acting beta-2 agonists (LABAs), salmeterol and formoterol have duration of action of approximately 12 hours with unknown mechanism. Because of their specificity, the patients experience fewer palpitations, tachycardia or tremor. There is only mild tachyphylaxis on regular use. However, the bronchoprotective effect of LABAs rapidly diminishes with regular use. In general, the effectiveness of SABAs is not impaired with regular use of LABAs. SMART study published in 2006 raised the concerns about the safety of LABAs in children and adults (especially in African-Americans), with increased risk of severe exacerbations and deaths when used with other asthmatic treatment.28 Subsequent literatures suggested that LABAs should not be used as a monotherapy. It should be used in combination with inhaled ICSs and only in situation after failure of optimal dose of ICSs as maintenance therapy. Combination inhalers are preferred over individually prescribed devices.29 A meta - analysis in 2010 showed that for adolescents and adults with uncontrolled asthma who were on low dose ICS monotherapy, the combination of LABA and ICS was more effective in reducing the risk of OCSs treated attacks than a higher dose of ICS. Better response was noted in terms of lung function, symptom control, use of bronchodilators and lesser withdrawals due to poor control. The treatment appears relatively safe in adults. However, in children, combination therapy is associated with a trend towards an increased risk of exacerbations requiring OCSs and hospital admissions. The safety of combination therapy in children under the age of 12 years becomes a concern.30 A more recent meta-analysis in 2005 that recruited only asthmatic children showed that, the combination of LABA to ICS resulted in improvement in various lung function parameters. However, it did not result in a significant reduction in the OCS treated attacks. The study also revealed a trend towards increased risk of hospital admission with LABA (although not statistically significant), irrespective of the dose of ICS.31 For children below 5 years of age, there was only few data on the use of LABAs. 3. Systemic corticosteroidsLong-term treatment with OCS, for periods longer than two weeks, may be required for severe uncontrolled asthma. Its use may be associated with significant adverse effects. Because of the side effects of prolonged use, OCS in children with asthma should be restricted to the treatment of acute severe attacks. Even short-courses of OCS, if used repeatedly, increase the risk of complications. 4. Leukotriene receptor antagonists (LTRAs)Singulair is the only recommended LTRA to be used in children. Its mechanism of action is by inhibition of the binding of cysteinyl leukotrienes to cysteinyl receptors on inflammatory cells and airway smooth muscle. Asthma management guidelines recommend using LTRAs as an alternative therapy for mild persistent asthma in patients who are unable or unwilling to use ICSs. LTRAs have the advantages of ease of use and high rates of compliance. In comparison with ICSs, LTRAs may have fewer adverse side effects but it is not as effective as ICSs when use alone.32 In children with inadequate asthmatic control on low doses of ICS, asthma management guidelines recommend adding LTRAs to ICS as one of the alternative options. Although the Cochrane Review in 2011 showed that the addition of LTRAs to ICS brings modest improvement in lung function33, there is no updated evidence to support the efficacy and safety of this combination especially in children. There is no evidence that the combination therapy will decrease the need of OCSs use or hospitalisation rate in comparison with the same or an increased dose of ICS in children and adolescents with mild to moderate asthma.34 For adverse effects of LTRAs, there is a slight increase in the rate of (rare) neuropsychiatric disorders. LTRA vs LABAsIn comparison between LTRAs and LABAs, evidences revealed that in adults with asthma inadequately controlled by low-dose ICS, the addition of LABA to ICS resulted in better responses in terms of symptoms and quality of life, bronchodilator and OCS use, and lung function parameters. However, only few paediatric data is available for firm conclusion.35 5. Anti-IgE (Omalizumab)Omalizumab is an anti-immunoglobulin E (anti-IgE) recombinant humanised monoclonal antibody. It works by binding to the Fc portion of the free IgE and hence interfere its binding to the IgE receptors and down-regulate its expression on mast cells and basophils. Subsequently, it affects the cell activation and mediator release.36 It is given subcutaneously by injection every two to four weeks. The asthma guidelines have recommended omalizumab for use as add-on therapy in adults and children over six years of age with inadequately controlled severe persistent allergic IgE-mediated asthma who require continuous or frequent treatment with oral corticosteroids. Systematic review with meta-analysis in adult and children showed that the adjunctive therapy was effective in reducing asthma exacerbations and hospitalisations and more patients were able to reduce or withdraw their inhaled steroids. The patients also noticed to have improved asthmatic symptoms and quality of life. There were few side effects except skin reactions at the injection site.37 Another systematic review on asthmatic children and adolescent with moderate-to-severe persistent severity also revealed similar efficacy and safety profile.36 Stepwise approach to asthma in children (Figure 1)To assess one’s asthma control is to measure the reduction of impairment and risk by intervention. Ideally, one should aim at ‘complete control’ of the disease which is defined as no daytime symptoms, no nocturnal awakening, no need for rescue bronchodilator, no asthma exacerbations, no limitations on exercise and daytime activity, normal lung function parameters and minimal side effects of drug. For the approach of asthma management, appropriate treatment algorithm should be commenced according to the patients’ pretreatment severity in order to achieve early control. Step up the treatment if control is not satisfactory. Step down the treatment when good control is achieved. Before considering to step up the treatment, review the compliance of the patients, their technique in proper use of inhaler and the control of triggering factors. On stepdown, decide which drug to decrease first and the rate by taking into consideration of asthma severity, the side effects, the dosage, the beneficial effect achieved, and the patient’s preference. If ICS is used, the lowest possible dose should be aimed at. Decrease the dose of ICS by approximately 25-50% every three months in outpatient clinic. One should aware that exercise-induced asthma usually reflects poor control and controller medications should be reviewed. 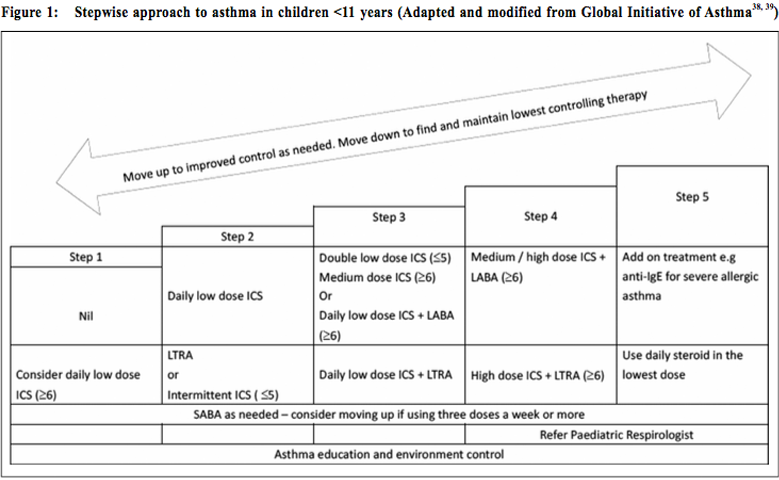
There are some differences regarding the recommendation for medications among children ≤ 5, children 6-11, and adolescents (adults).
ConclusionAsthma remains the most common chronic disease in childhood. Its prevalence is up to 10.2% of children in Hong Kong. There is no gold-standard diagnostic test for asthma and its diagnosis remains a clinical judgement. Asthma is a disease characterised by chronic airway inflammation. It is defined by the history of episodic respiratory symptoms with variable airflow limitation. Successful treatment of asthma involves three components: controlling and avoiding asthma triggers; monitoring asthma symptoms and lung function regularly; and understanding how and when to use medications. Inhaled corticosteroid remains the mainstay of treatment of childhood asthma. However, steroid phobia is a very common issue in Chinese community.40 A recent local survey showed that if asthma was not under good control with suboptimal dose of inhaler therapy, many people used Complementary and Alternative Medicine (personal communication with Professor Ellis KL Hon, Department of Paediatrics, The Chinese University of Hong Kong). Detailed discussion about proper use of medications with their parents will be necessary. The severity of asthma can be classified into intermittent and persistent (mild, moderate and severe). A stepwise approach and an individualised treatment plan should be adopted. The aim of asthma management is to control the disease. Complete control is defined as no daytime symptoms, no nocturnal awakening, no need for rescue bronchodilator, no asthma exacerbations, no limitations on exercise and daytime activity, normal lung function parameters and minimal side effects of drug. 
Wa-keung Chiu, MBBS(HK), FHKCPaed, FRCPCH
Correspondence to: Dr Wa-keung Chiu, Department of Paediatrics and Adolescent
Medicine, United Christian Hospital, Hip Wo Street, Kwun Tong, Kowloon, Hong Kong SAR.
References:
|
|