|
March 2016, Volume 38, No. 1
|
Original Article
|
Prevalence of different severities of chronic obstructive pulmonary disease in an out-patient clinic in Hong KongSze-wai Yeung 楊詩煒,Pang-fai Chan 陳鵬飛,Loretta KP Lai 黎潔萍,Kai-lim Chow 周啟廉,Matthew MH Luk 陸文熹, David VK Chao 周偉強 HK Pract 2016;38:3-12 Summary
Objective: (1) To evaluate the prevalence of different
categories of Chronic Obstructive Pulmonary Disease
(COPD) severity in a general outpatient clinic (GOPC)
using a combined assessment method recommended
in the Global Initiative for Chronic Obstructive Lung
Disease (GOLD) 2013 guideline and (2) to describe if
any difference in the categorisation of COPD patients
by using COPD Assessment Test (CAT) versus Modified
Medical Research Council Dyspnoea Scale (mMRC) and
also (3) to investigate the adherence of pharmacological
treatment to the guideline.
Keywords: chronic obstructive pulmonary disease, COPD assessment test, Modified Medical Research Council Dyspnoea Scale, COPD categorisation, primary care 摘要
目的:根據GOLD 2013指引(Global Initiative for Obstructive
Lung Disease),評估普通科門診按不同嚴重程度分類COPD
的發病率,並且比較使用COPD評估測試(CAT)和醫學
研究理事會改良呼吸困難量度表(m M R C)對C O P D 病
人分類的異同。另外就藥物治療對於指引的依從性進行了
研究。
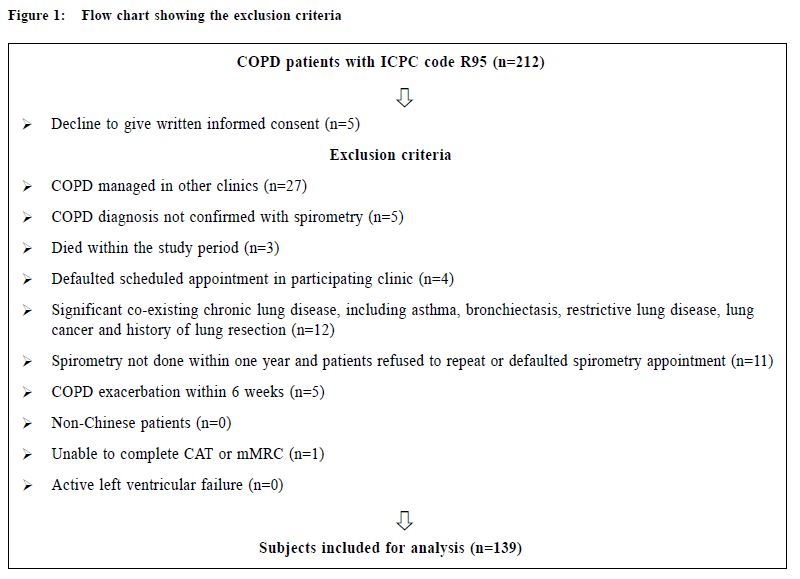
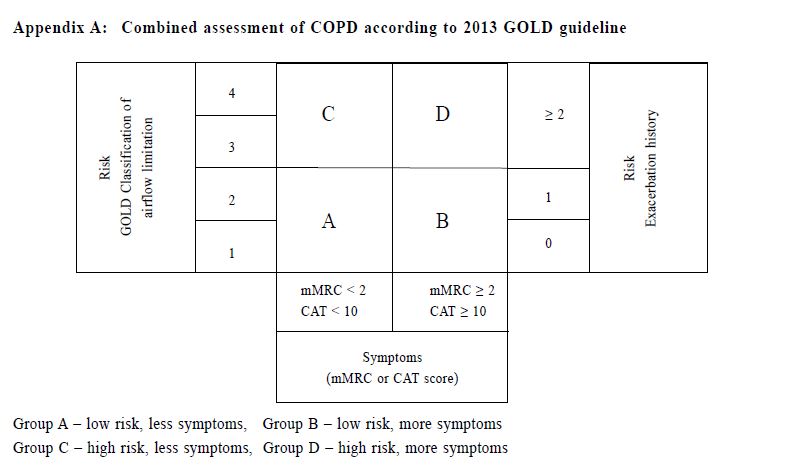
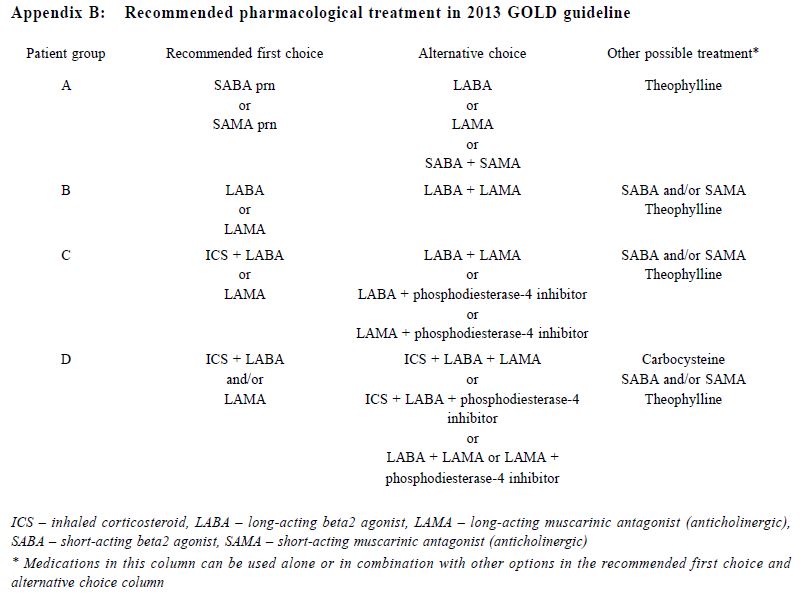
關鍵詞:慢性阻塞性肺疾病,慢性阻塞性肺病評估測試, 醫學研究委員會改良呼吸困難量度表,COPD分類,基層醫療 Introduction Chronic Obstructive Pulmonary Disease (COPD) is an important global health problem. According to the World Health Organisation, an estimated 64 million people worldwide had COPD in 2004, with more than 3 million deaths attributed to COPD in 2005.1 COPD was also the third leading cause of death globally in 2012.2 One study in Hong Kong suggested that 9% of people aged over 70 years suffered from COPD.3 The prevalence of COPD in Hong Kong was estimated to be 3.5% in 2000.4 In 2012, COPD was the cause of over 31,000 hospitalisations in public hospitals and was the fifth leading cause of death in Hong Kong.5 COPD patients are also commonly encountered in general outpatient clinics (GOPC). In our public primary care system, COPD ranked the seventh commonest chronic medical disease in 2013. By improving the standard of care of COPD patients provided in the primary care, the disease morbidities including the number of admissions due to COPD exacerbations may be reduced. The 2011 Global Initiative for Chronic Obstructive Lung Disease (GOLD) guideline introduced the categorisation of COPD patients into 4 groups using the combined assessment of symptoms (using the COPD assessment test [CAT] or the modified Medical Research Council [mMRC] dyspnoea scale), airflow limitation using spirometry and risk of exacerbations to improve disease management (Appendix A).6 The mMRC scale (a range of from 0 to 4) was developed by the American Thoracic Society as a modification of the original British Medical Research Council dyspnoea index, and is used to grade the degree of disability due to breathlessness, with 4 representing the most severe category. The CAT consists of 8 items with an overall score from 0 to 40. According to the GOLD 2013 classification, patients were classified with either mMRC score (0–1 versus ≥2) or CAT score (<10 versus ≥10) resulting in two low-symptom categories (A and C) and two high-symptom categories (B and D). Exacerbation risk was assessed with either forced expiratory volume in one second (FEV1) percentage predicted (<50% versus ≥50%), or COPD exacerbation history (0–1 versus ≥2) in the previous one year to stratify patients into low-risk groups (A and B) and high-risk groups (C and D). This categorisation provides a guide for evidence-based pharmacological treatment , with an aim to reducing symptoms , improving health status and reducing exacerbation.6 CAT is a multidimensional questionnaire assessing different symptom domains and health status related to COPD. It was shown to correlate with some clinically important variables including FEV1 and exacerbation frequency. It has high sensitivity and repeatability.7-10 On the other hand , mMRC is a unidimensional symptom scale assessing only the degree of disability due to dyspnoea. mMRC is widely used clinically because of its simplicity and long history of establishment.11-13 The difference in nature between these two symptom scores makes their application in the GOLD combined assessment in doubt. The prevalence of different COPD categories was studied in a pulmonology clinic in Korea with highest prevalence in group D by using CAT score and highest prevalence in group A by using mMRC scale.14 This study showed a moderate agreement for the GOLD categories by using CAT score and mMRC scale (κ= 0.510).14 In a recent study in China involving pulmonary clinics patients, the CAT score was also shown to be only moderately correlated with the mMRC scale (ρ= 0.579).15 Other international studies also showed that there was significant heterogeneity in group assignment by using different symptom scores.16-18 The combined assessment of COPD was not widely adopted in our GOPCs. Published data about the prevalence of different COPD severity categories and the correlation of CAT and mMRC in primary care in the local or international setting are lacking. Understanding our local prevalence will be useful for devising future policies on COPD management in Hong Kong’s primary care. Drug choices for COPD treatment are limited in GOPCs as most patients were thought to be having mild diseases. Newer medications including longacting beta2-agonists (LABA), long-acting muscarinic antagonist (LAMA) and combined long-acting beta2- agonits/inhaled corticosteroids (LABA/ICS) are mostly unavailable in GOPCs. According to the 2013 GOLD guideline, some of the newer drugs were recommended as first line treatment among group B, C and D patients (Appendix B).6 Studies showed that the level of adherence to guidelines in the management of COPD has been consistently unsatisfactory.19-22 Understanding the level of adherence to treatment guideline in our local clinic is important to identify the service gap for quality improvement. The primary objective of our study was to evaluate the prevalence of different COPD severity categories as defined by the 2013 GOLD guideline in our GOPC. The secondary objectives were to describe the differences in the COPD severity categorisation between CAT score and mMRC scale, and to compare the pharmacological treatment provided in the clinic with the recommended treatment in 2013 GOLD guideline. Method Study design This was a cross-sectional study carried out in a GOPC in Hong Kong. Patients assigned with the International Classification of Primary Care (ICPC) code R95 (Chronic Obstructive Pulmonary Disease) and followed up in our clinic from 1st January 2014 to 31st May 2014 were identified from the Hospital Authority’s Clinical Data Analysis and Reporting System (CDARS). These patients were invited to participate in the study when they attended for their follow up. After obtaining informed consent, all patients would receive combined assessment according to the 2013 GOLD guideline. The flow chart in Figure 1 illustrates our inclusion and exclusion criteria. This study was approved by our Kowloon Central Cluster/Kowloon East Cluster Research Ethics Committee/Institutional Review Board.
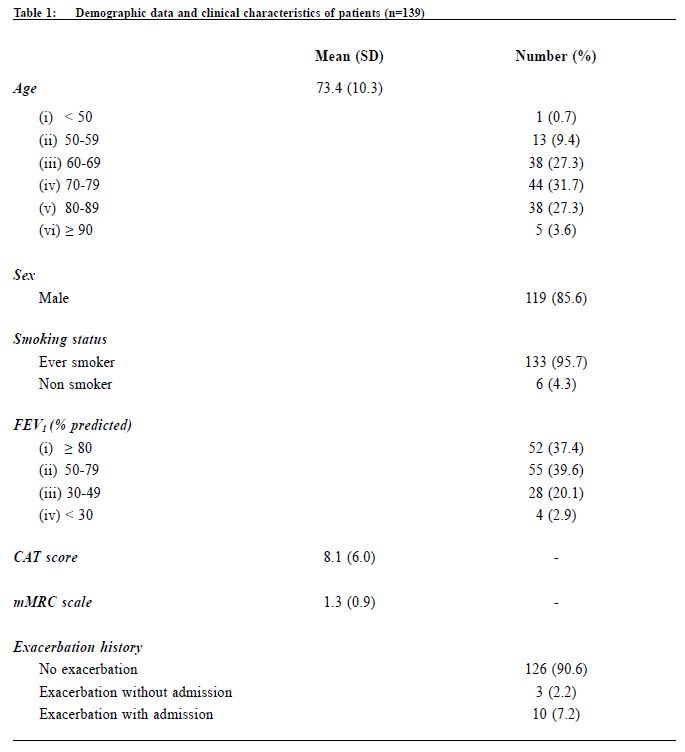
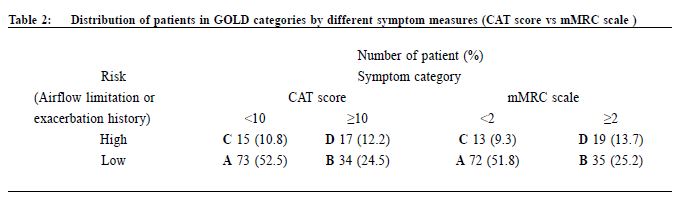
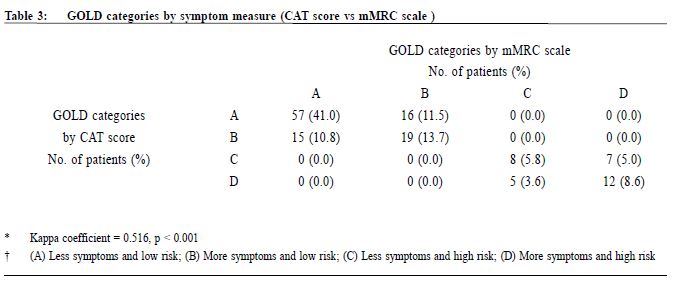
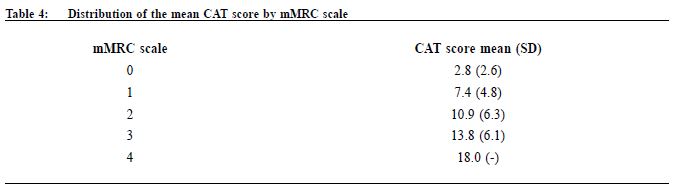
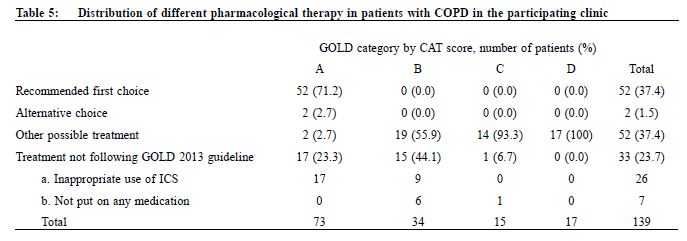
Procedure A questionnaire including the CAT score and mMRC scale was administered by trained nurses during patient follow-up. Both the English and validated Chinese versions were available.23-25 The number of exacerbations in the previous one year was obtained from patients’ history at the same consultation when mMRC scale and CAT score were measured. An exacerbation was defined as an acute event with worsening of the patient’s respiratory symptoms that is beyond normal day to day variations and leads to a change in medication.6 Patients would be referred to repeat a spirometry assessment if the previous results were more than 1 year from the study period. The validated hand-held spirometer Spirolab III was operated by a trained nurse for lung function testing - using spirometry results from adult Hong Kong Chinese data as reference. According to the 2013 GOLD guideline , pharmacological management of COPD is classified into recommended first choice, alternative choice and other possible treatments . 6 Participants’ medical records were reviewed for comparing with recommended management. Statistical analysis All statistical analyses were performed with IBM SPSS version 21.0. Proportions were presented as percentages. Continuous data with normal distribution were presented by mean with standard deviations. Kappa coefficient (κ) and Spearman correlation (ρ) was used to examine the extent of agreement and correlation between CAT versus mMRC score respectively. Differences were considered statistically significant if p < 0.05. Results Study population 212 patients were coded as having COPD in our clinic. 5 patients refused to participate in the study and 68 patients were excluded due to various reasons as shown in Figure 1. As a result, 139 subjects were included for data analysis. Clinical characteristics of the subjects were summarised in Table 1. 90.6% had no exacerbations over the past year. 7.2% experienced exacerbations requiring admission. Prevalence of different GOLD categories The prevalence of different GOLD categories is shown in Table 2. Using the CAT score, the prevalence of categories A, B, C, D were 52.5%, 24.5%, 10.8% and 12.2% respectively. On the basis of the mMRC scale, the prevalence of categories A, B, C, D were 51.8%, 25.2%, 9.3% and 13.7% respectively. The prevalence of group A (less symptoms, low risk) was the highest, and least patients were classified as group C (less symptoms, high risk) irrespective of the assessment tools used. Correlation of the GOLD categories between CAT score and mMRC scale There was moderate agreement for the GOLD categories by using CAT score and mMRC scale with kappa coefficient of 0.516 (p<0.001) (Table 3). The Spearman’s correlation coefficient for CAT score and mMRC was 0.572 (p<0.001), suggesting moderate correlation. The cut-point for mMRC (score of 2) corresponded with a mean CAT score of 10.9 which was approximate to the cut-point (score of 10) of CAT (Table 4). Adherence to the recommended pharmacological treatment Data analysis in this part was performed according to CAT score categorisation because available evidence suggests CAT is more repeatable and sensitive than the mMRC scale.7,8 Among the 139 subjects, 37.4% were put on the recommended first choice treatment. 1.5% and 37.4% of them were put on alternative choice and other possible treatments respectively. The pharmacological treatment provided in 23.7% of the subjects was not following any of the recommended options (Table 5). The reasons for this were prescription of ICS (78.8%, 26/33) in low risk patients and no medication given (21.2%, 7/33) in indicated patients. 71.2% (52/73) of category A patients were put on first choice treatment, while 5.5% (4/73) were on alternative or other possible treatments. In category B, C and D, no patients were put on first or alternative choice treatment. 55.9% (19/34) of category B patients, 93.3% (14/15) of category C patients and 100% (17/17) of category D patients were treated with medications belonging to other possible treatments. Up to 23.3% (17/73) of category A patients, 44.1% (15/34) of category B patients and 6.7% (1/15) of category C patients were not receiving pharmacological treatment in accordance to the 2013 GOLD guideline.
Discussion Prevalence of different GOLD categories Our results showed that our clinic has a high prevalence (77%) of categories A and B patients and among these groups of patients, nearly one-third belonged to group B, i.e. more symptoms. There was also a significant proportion (23%) of patients with high COPD exacerbation risk (categories C and D), highlighting the importance of performing comprehensive assessment for these patients in order to optimise their management and reducing their risk. Categorisation by using CAT score and mMRC scale Our results are consistent with existing literature, demonstrating heterogeneity in COPD category assignment with different symptom scores14,16-18, and only moderate correlation between CAT score and mMRC scale.14-18 Although the mMRC scale continues to be recommended in the 2014 GOLD guideline, the CAT score is preferred because of its more comprehensive assessment.28 However, in our busy primary care setting, the mMRC scale remains a useful alternative to CAT score. Moreover, our results confirm that a mMRC score of 2 can be used as the cut-point as recommended in the 2013 and 2014 GOLD guidelines.28 In order to ensure continuity of care, patients' symptoms should be monitored with the same assessment tool (either CAT or mMRC) for COPD categorisation. Adherence to the recommended pharmacological treatment In our study, majority of our patients were receiving pharmacological treatment according to the recommendations in 2013 GOLD guideline. However, except in group A, all patients in other groups were receiving medications belonging to other possible treatment instead of recommended first choice or alternative choice. Selected choices of COPD drugs in GOPC drug formulary may be one of the reasons for this observation. Existing evidence suggests that long-acting bronchodilators are preferred over shortacting bronchodilators in the management of COPD.29,30 The benefits of LAMA, LABA or LABA/ICS including long-term improvements in lung function, quality of life, and reduction of exacerbations in patients with COPD were well demonstrated in international studies.30,31 Inclusion of long-acting bronchodilators and LABA/ICS in the GOPC drug formulary may be beneficial in improving the care of some COPD patients. The cost-effectiveness of introducing these medications in GOPC would require further studies. ICS is recommended for patients with FEV1 less than 60% predicted. However, the prescription of ICS in some low risk group patients was observed in this study. Similar finding was found in other western countries.32,33 Unfamiliarity with the latest GOLD guideline has been suggested as one of the reasons for this finding.32,34 Another reason could be due to inconsistency of recommendations among different guidelines. According to the National Institute of Clinical Excellence (NICE) 2010 guideline for COPD management, in people with stable COPD and an FEV1 of more than 50% who remain breathless or have exacerbations despite maintenance therapy with a LABA, LABA plus ICS in a combination inhaler could be considered.35 Therefore, doctors may add ICS to the low risk group patients because of the unavailability of long-acting bronchodilators. Limitations We acknowledge some limitations in our study. Firstly, some patients with recent COPD exacerbation were excluded from the study and hence the true prevalence of patients at higher exacerbation risk would be underestimated. Secondly, subjects were recruited from a single primary care clinic which limits generalisability of results to other primary care clinics, although our results were comparable to other international multicenter studies. Thirdly, our subjects were predominantly male, and so our findings might not be applicable to female COPD patients. Lastly, some ICS was initiated in patients by the previous attending physicians for various clinical indications, which might include suspected or tested airway reversibility and after acute exacerbations before referring to GOPC for follow-up. Airway reversibility was also not tested by spirometry in the participating clinic. As a result, the assessment of non-adherence in currently low risk patients would be overestimated. In the future, multi-centered prospective studies on the application of the combined assessment in COPD patients on long term follow up could be of significant clinical importance in investigating the practicability and benefits of integrating the recommended symptoms and risks stratification in primary care.
Conclusion In our study, the prevalence of GOLD categories A and B was the highest but there was a significant proportion of patients at high risk of COPD exacerbation. There were significant differences in GOLD categories assignments between CAT score and mMRC scale. Therefore, COPD patients' symptoms should be monitored with the same symptoms assessment tool in order to preserve continuity. mMRC scale would be a suitable alternative in assessing the degree of dyspnoea when CAT score is not clinically applicable. The observations of underuse of long-acting bronchodilators or combined LABA/ICS and overuse of ICS were highlighted in this study. The addition of those unavailable recommended first choice medications into the GOPC drug formulary is recommended. Acknowledgement The authors wish to express their gratitude to the nursing staff in the participating clinic for their assistance in obtaining patients’ consents and completing the questionnaires, and also the doctors in the participating clinic in the recruitment of eligible patients.
Sze-wai Yeung, FHKAM, FHKCFP, FRACGP
Resident Pang-fai Chan, FHKAM, FHKCFP, FRACGP Consultant Loretta KP Lai, FHKAM, FHKCFP, FRACGP Associate Consultant Kai-lim Chow, FHKAM, FHKCFP, FRACGP Associate Consultant Matthew MH Luk, FHKAM, FHKCFP, FRACGP Associate Consultant David VK Chao, MBChB (Liverpool), MFM(Monash), FRCGP, FHKAM (Family Medicine) Chief of Service and Consultant Department of Family Medicine and Primary Health Care, United Christian Hospital and Tseung Kwan O Hospital, Kowloon East Cluster, Hospital Authority, Hong Kong SAR, China. Correspondence to: Dr Sze-wai Yeung, 99 Po Lam Road North, Tseung Kwan O, New Territories, Hong Kong SAR, China. E-mail: ysw476@ha.org.hk
References
|
|