June 2012, Volume 34, No. 2 |
Original Articles
|
||||||||||
An evidence-based clinical audit on smoking cessation management for patients with chronic diseases in a Hong Kong general outpatient clinicL Lo 盧令,David VK Chao 周偉強 HK Pract 2012;34:66-75 Summary Objective: To evaluate smoking cessation management for patients with chronic diseases in a Hong Kong public primary care clinic by conducting a clinical audit, with an aim to induce change and improvement in service provision. Design: The first phase of the audit (7/2007 to 6/2008) was a retrospective medical record review that identified potential areas for enhancements in smoking cessation service. The second phase (7/2008 to 6/2009) was a prospective audit period implementing the improvement changes. Results from the two phases were compared. Subjects: All patients attending the Day, Evening, Sunday and Public Holiday clinics with one or more of the following six most common chronic diseases namely, hypertension (HT), diabetes mellitus (DM), asthma, chronic obstructive pulmonary disease (COPD), ischaemic heart disease (IHD), or cerebral vascular accident (CVA). Main outcome measures: The data from the 2 audit phases were measured against 5 audit criteria (Ask, Advice, Assess, Assist, Arrange) to look for improvement across the 2 phases and whether the final results were able to meet pre-set standards. Results: Statistically significant improvements were observed in criteria 1 to 3. Only criterion 5 could achieve the pre-set standard of 100%. Conclusion: A clinical audit with a targeted team approach to implement changes was capable of achieving significant improvements in the process of care for smoking cessation. However, systems limitations and human factors did exist preventing clinical performance to achieve pre-set standards. Keywords: Smoking, smoking cessation, clinical audit,chronic disease, evidence-based health care
摘要 目的:在本港一所公立基層醫療診所進行臨床審計,評估對慢性疾病患者所提供戒煙服務的質素,以進行改善措施。 設計:臨床審計分兩段為期一年的時間進行。第一階段(7/2007至6/2008)是將醫療記錄作回顧性分析,以確定在戒煙服務上的潛在改善空間。第二階段(7/2008至6/2009)是進行改善措施並作前膽性審計。最後,分析及比較兩個階段中的服務質素,以研究作進一步改善。 對象:在日診、夜診、星期日及公眾假期診所求診的六種常見慢性疾病患者,包括所有高血壓、糖尿病、哮喘、慢性阻塞性肺病、缺血性心臟病及中風的病人。 主要測量內容:衡量在兩個階段數據中的五項審計準則:詢問、建議、評估、協助、安排,以便確定質素是否得到改善,並評估最終成果能否達到預定標準。 結果:戒煙服務中的首三項審計準則有顯著改善,而第四及第五項審計準則因牽涉及人數較少,其改善程度未能確定。於五項審計準則中,只有第五項能夠達到預定的100%標準。在是次為期一年的臨床審計,4位病人能夠成功戒煙,41位病人獲轉介至戒煙中心跟進。 結論:在指定團隊以臨床審計方法推行改善措施,能顯著改善戒煙服務質素。然而,制度上的限制及人為因素的存在,都阻礙了達至臨床上的預期標準。 主要詞彙:吸煙、戒煙、臨床審計、慢性疾病、實證醫學醫療。
Background Smoking is arguably the most preventable cause of deaths in Hong Kong (HK) and in many other countries. Indeed, tobacco use is a risk factor for 6 of the 8 leading causes of deaths globally.1 The World Health Organization estimates that smoking kills nearly 6 million people a year, and approximately 1 person dies every 6 seconds due to tobacco accounting for 1 in 10 adult deaths. Up to half of all current users will eventually die of a tobacco-related disease. As at 2010-2011, the prevalence of smoking in HK is approximately 12.0 - 13.6%. Smokers consume an average of 13.4 cigarettes per day. Of the smokers, 68.8% are aware of local smoking cessation services but only 2.5% have tried them before.2-3 A local study4 in 2006 estimated that among those, in 1998 who were aged >35 years, 5,596 and 1,324 deaths were attributed to active and passive smoking respectively. In 2000, almost 34,500 people were hospitalized from smoking related illnesses. For adults, the attributable cost of public hospital use was $230 million a year, representing 7% of the Hospital Authority's total expenditure on public hospitals in 1998. The cost of visits to public primary care outpatient clinics was estimated at $21 million, representing 12% of the total cost for such clinics in 1998. Smoking poses a significant risk on health, regardless of age and sex. However patients with chronic diseases are especially at risk. The General Outpatient Clinics (GOPCs) of HK look after a large number of patients with chronic diseases, and are also the point of entry for many other walk-in patients. GOPCs are therefore at a pivotal position to intervene and to offer help to smokers. The principal investigator conducted a clinical audit on smoking cessation because the standard of care in his clinic had room for improvement: the lack of standardized guidance for clinicians on smoking cessation practice, the relative lack of nursing and clerical support, and an apparent low intervention (e.g. referral / anti-smoking prescription) rate observed. This situation is similar to the published data from another local primary care clinic.5 Objective The objective of the audit is to evaluate the process of care for smoking cessation intervention in the principal investigator’s clinic, with an aim to induce change and improvement in service provision:
Method Study design This clinical audit consists of 2 phases, with comparable setup and collection of statistical data. The first phase (1/7/2007 to 30/6/2008) is a retrospective review of records, followed by the identification of areas for enhancements. The second phase (1/7/2008 to 30/6/2009) is a prospective audit period implementing the improvement measures. After the second phase, a final analysis is performed to compare the performance in the 2 phases and to further investigate areas for future improvement. Medical records were reviewed to assess whether details relating to the 5 audit criteria were included in the records during the pervious 12 months.
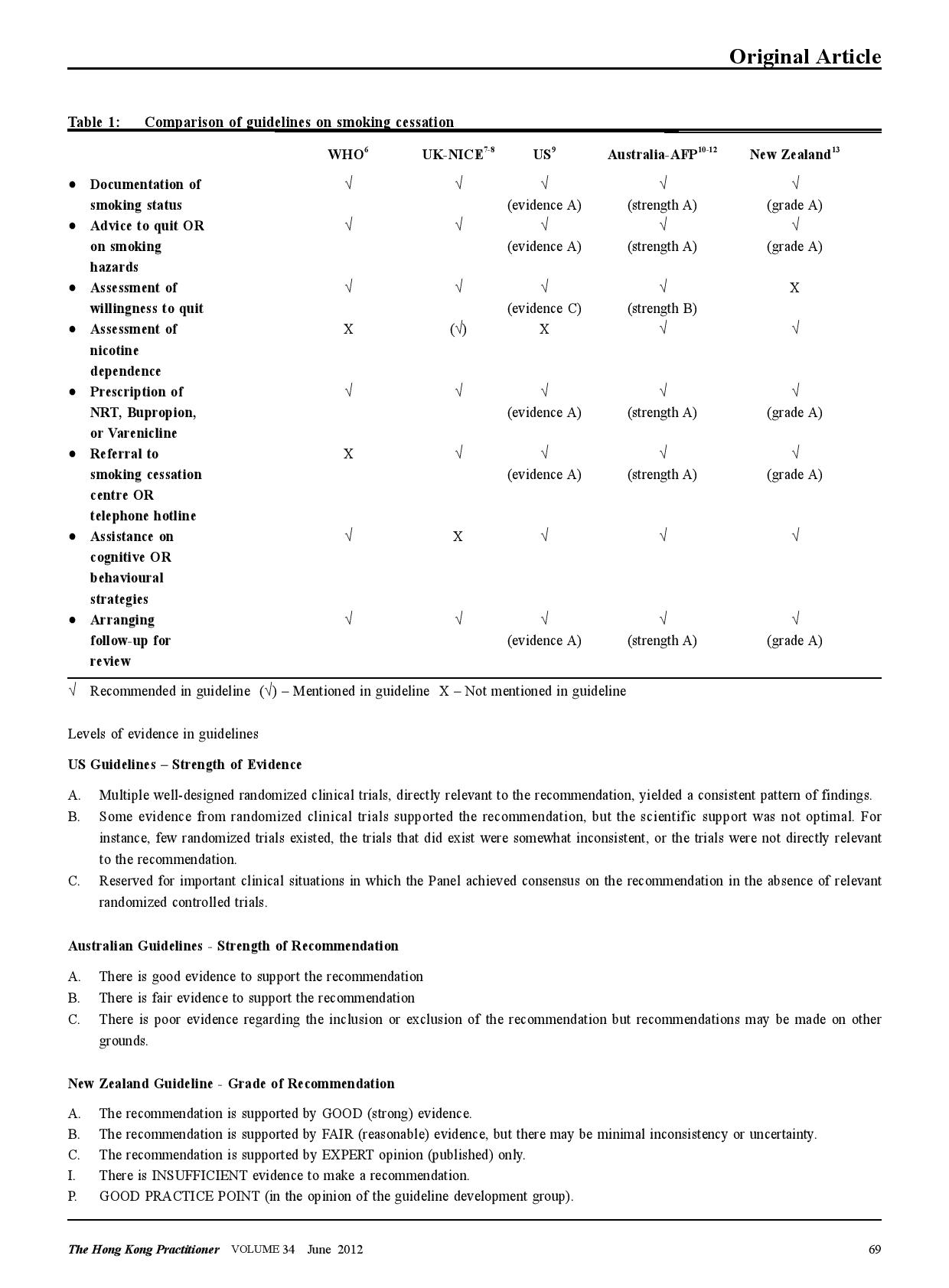
The evidence-based audit criteria A literature search in Pubmed, corresponding government authorities, academic colleges, and local institutions involved in smoking cessation services was undertaken to retrieve relevant clinical resources including previous audit reports, established clinical guidelines, and known standards of care. A number of clinical guidelines on smoking cessation have been released by various health authorities and professional organizations (Table 1).6-13 However, there was a paucity of local guidelines. The 5 criteria used in this audit are based on the 5A’s framework put forward by the US guideline.9 The guideline was selected because it provides the most comprehensive information on the evidences underpinning its recommendations. The 5 criteria are also well supported by the latest Cochrane reviews.14-18 Criterion 1 (ASK). This originates from the 2000 US Public Health Service clinical practice guideline: treating tobacco use and dependence.19 The evidence supporting this stems back from the meta-analysis in the 1996 US Guideline,20 where identifying smokers increases rates of clinician intervention. Although asking alone does not improve abstinence rate significantly, it forms the first step in providing any intervention to potential patients. Criterion 2 (ADVICE) . The US Guideline recommends that all physicians should strongly advise every smoker to quit because evidence shows that physician advice to quit smoking increases abstinence rates. The evidence originates from a meta-analysis of 7 studies in the 1996 US Guideline.20 The benefit of physicians’ advice is also supported by the latest Cochrane review in 2008,14 showing that simple advice has a small effect on cessation rate. Assuming an unassisted quit rate of 2 to 3%, a brief advice-intervention can increase quitting by a further 1 to 3%. It is also interesting to note that advice delivered by other health care providers also result in an increased quit rates.21 These support the team approach in intervening patients who smoke. Criterion 3 (ASSESS ) . The US guideline recommends that once a tobacco user is identified and advised to quit, the clinician should assess the patient’s willingness to quit. One systematic review shows that assessing the smokers’ motivation enables a stage-based smoking intervention, which is effective in changing smoking behaviour.22 For the principal investigator’s clinic , the availability of specialized smoking cessation service is limited. Assessing the willingness to quit enables the clinician to stratify the more eager patients who would benefit most. Criterion 4 (ASSIST). Assisting encompasses a range of cognitive and behavioural strategies offered by the clinician. Only prescriptions and referrals are audited in this exercise. The efficacy of the 3 first-line medications for smoking cessation (nicotine replacement therapy or NRT, Bupropion, and Varenicline) is well evidenced by the Cochrane reviews,15-17 the NICE review23 and the 2008 US Guideline. The US guideline shows that combining medication and counseling is better than medication alone or counseling alone. Currently, combining NRT prescription and smoking counseling are not a routine in GOPCs, but is part of the services provided in the specialized Smoking Counseling and Cessation Centres (SCCCs). Criterion 5 (ARRANGE). The US guideline recommends that all smokers should be assessed with respect to their smoking status during follow-up clinical contacts of all patients . In particular, assessments within the first week after quitting should be encouraged. This recommendation is based on 2 studies.24-25 However, the existing evidence does not show that these steps will prevent relapse, but continued involvement on the part of the clinician may increase the likelihood that the patient will consult the clinician in later quit attempts should they be needed.
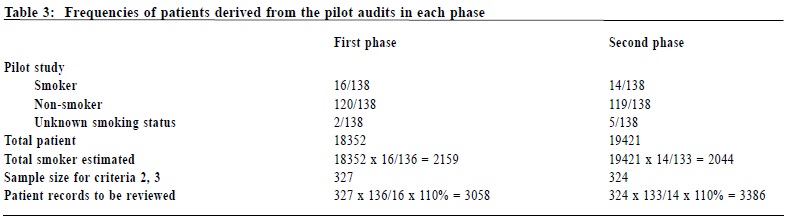
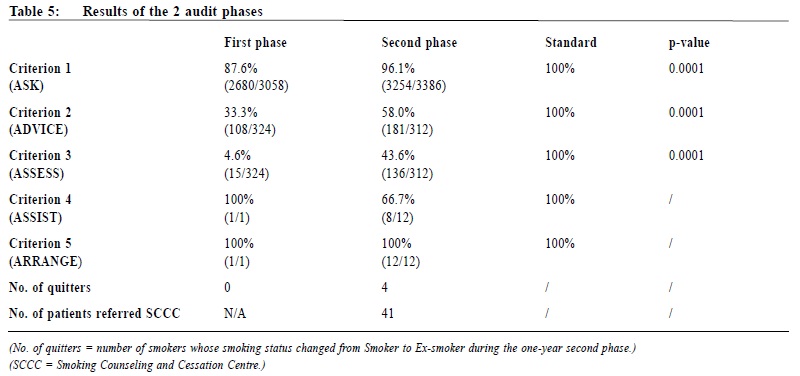
Setting of standards The setting of standard for each criterion has taken into consideration known standards published by a local audit5 in 2005, and an audit protocol by the National Institute for Health and Clinical Excellence (NICE) of the United Kingdom in 200626 (Table 2). Subjects The target population (grand audit population) in this audit is all patients attending the author’s clinic with any of the 6 commonest chronic diseases, namely hypertension (HT), diabetes mellitus (DM), asthma, chronic obstructive pulmonary disease (COPD), ischaemic heart disease (IHD), and cerebral vascular accident (CVA), and who are at least seen once by one of the regular resident doctors in the clinic. Patients with the specified diagnoses who had only been seen by locum / relieving doctors were excluded because of the potentially wide variation in practice among this group of doctors. Sample size calculations The grand target population has been defined above. However, the “sub-population”, and therefore the sample size required, for each of the 5 criteria is different: For criterion 1 (ASK), the population is the total number of eligible chronic patients. It is approximately 18,000 in each phase. From previous local audit data5 and clinical experience, the percentage of smoking status documentation is roughly 90%. Therefore, assuming that 90% of the patients would meet the criterion, the sample size required at 95% confidence level with 95% confidence interval (85-95%) is estimated to be 138. For criteria 2 (ADVISE) and 3 (ASSESS), the population is the total number of smokers. In the retrospective first phase, this number was unknown as there was not a complete list of smokers. One solution was to retrieve and audit those patients coded27with Tobacco Abuse in the clinic computer system. However, there were only 348 such coded records. This low coding rate meant that the coded list of smokers was not representative of the total number of smokers. Many smokers were simply un-coded. In the second phase, the total number of smokers was again unknown. So, in order to find the total number of smokers, the principal investigator underwent a pilot audit. The Pilot and Main Audits. From the review of the initial sample of 138 (above), it was found that there were 16 smokers, 120 non-smokers, and 2 patients with unknown documentation status in the first phase. The rough percentage of smokers in the clinic was ~12% (= 16/136). Therefore with the known grand population of 18,352 in the first phase, the total number of smokers was roughly 2,159 (= 18,352 x 12%) (Table 3). In criteria 2 and 3, the expected percentage of patients intervened (Advised or Assessed) is uncertain. It is prudent to assume a 50% chance to arrive at the largest possible sample size. With a target population of ~2159, assuming that 50% of the patients would meet the criterion, the sample size required at 95% confidence level with 95% confidence interval (45-55%) is estimated to be 327. As there was not a complete list of all smokers, those 327 smokers were extracted from the grand population. Here 2,780 (= 327 / ~12%) random patients from the grand population were required. Allowing a 10% safety margin, 3,058 (2,780 x 110% = 3,058) records were ultimately reviewed to recruit the 327 smokers. A similar pilot audit was also performed for the second phase to find the total number of smokers. For criteria 4 and 5, the population is theoretically the total number of smokers who are eager to quit.This figure is expected to be small. And even smaller is the actual number of patients who have been intervened. Statistical comparison of the 2 phases is less meaningful, and therefore the actual number of patients intervened is presented instead. Data extraction and collection The grand populations of eligible chronic patients are trieved from the clinic computer system. The lists of samples of patients for review are randomly generated by a custom-made randomization Macro in Microsoft EXCEL. They are then reviewed by the principal investigator against the 5 audit criteria. Computerized records are the standard operational notes used in the clinic, and so they were the main documentation reviewed. In case of uncertainty, the paper records were also reviewed. Interventions Identification of deficiencies and implementation of changes After the completion of the first phase, a systematic review was undertaken by the principal investigator to identify the deficiencies in the process of care. The principal investigator also sought advice from his senior colleagues and the clinic doctor-in-charge. The identified areas for enhancements and improvement changes are summarized in Table 4. After identifying the areas for enhancement, the principal investigator discussed with his clinic supervisor on the proposed improvement measures. A clinic meeting involving the principal investigator, the clinic supervisor, the nursing officer, a nurse representative, a dispenser, and a member of the clerical staff was held to introduce the importance of smoking cessation activities. The initial findings of the first phase, the setup of the audit, and the proposed improvement changes were discussed. The consensus of the meeting was then relayed to all nursing, supporting and clerical team members. A formal invitation letter detailing the audit design was sent to all other medical staff of the same department to seek their assistance on the project. A second reminder was also delivered to each Evening, Sunday, and Public Holiday clinic relieving doctor when he / she first came back to the clinic for relieving duties. Furthermore, during the review of the first phase data, it was noted that there had been a wide range of clinical performance on smoking cessation intervention within the medical team. The principal investigator then approached his colleagues individually to relay the importance of anti-smoking activities, to discuss any barrier to practice, and to seek their active participation in the second phase. Outcome measures The outcome measures in this audit refer to improvement achieved across the 2 phases, and whether the final results met pre-set standards. Statistical methods Chi-square (without Yate’s correction as the sample sizes are large) is employed to evaluate statistical significance. Two-tailed p values are used to demonstrate statistical significance. For criteria 4 and 5, the actual numbers of subjects are small. Therefore, the actual frequencies are presented. Results The results of the 2 audit phases are summarized in Table 5. In both phases, criteria 1 to 3 did not meet pre-set standards, although there were statistically significant improvements. Criterion 4 shows an apparent drop in the performance, but that could be attributed to the very small number of subjects. Across the 2 phases, there is a large increase in the number of eager smokers identified (from 1 to 12). It is due to an enhanced assessment of the willingness to quit. For the actual number of patients quitted smoking during the audit period, there is none in the first phase while there are 4 in the second phase. For the total number of smokers referred to a smoking cessation centre, there is no data available in the first phase, while there are 41 in the second phase as demonstrated in a prospective logbook. Discussion Why were some pre-set standards not met? The principal investigator ’s clinic is a large regional GOPC with evening, Sunday and public holiday clinics. Many patients who have defaulted appointment in their original district clinics come to have follow up in this clinic as the final resort. Many of them are seen only once or infrequently over a one-year period, and that leaves little opportunity for preventive activities. Furthermore, patients with stable chronic illnesses are increasingly given longer follow-up appointments. Longer follow-up intervals result in fewer encounters for opportunistic preventive interventions. A final reason for the inability to meet standards could be linked to human factors. During the data collection of the first phase, it had been noticed that there was a wide range of performance among different clinicians. This large deviation was again observed when the principal investigator reviewed the second phase records. An impression was that several clinicians were not performing according to expectation in the process of care. This impression of the principal investigator, however, cannot be quantified because patients are frequently seen by more than one clinician over the one-year period. Impact on patient care The benefits brought about by the implementation were not limited to service improvement, but also a change in attitude of all clinic staff. Many dedicated colleagues continued to keep updating patients’ smoking status, assessing patient’s willingness to quit or even referring patients to SCCC after the formal audit period has ended. This is indeed an atmosphere and motivation brought about by the audit, continuing the audit spiral.28 Limitations One limitation of this audit is that it is not easy to motivate locum doctors to adhere well to the audit protocol. The resident doctors of the principal investigator’s clinic are on average more motivated because there are more regular liaisons during the audit period. Another weakness of the audit is that the record reviewing process demands a high professional time input. Although the computer system is able to capture the smoking status of patients, it is not possible to draw such data by the frontline clinician without the help of the central information technology (IT) team. Currently, the reviewing process is mainly manually performed. Enhancement in computer system and close liaison with the IT team in planning would probably facilitate future audits. Future directions Further audits should be considered to assess smoking cessation management among patients without chronic illness, and also to review the management provided by nurses. Conclusions By completing a clinical audit cycle and combining a targeted team approach to implement changes, it is possible to bring about significant improvements in the process of care of smoking cessation intervention. However, systems limitations and human factors prevent clinical performance to approach pre-set standards. Further changes in care delivery are expected to result in further improvement. Further audits encompassing patients without chronic illnesses, or including nursing interventions, should be considered. Acknowledgement The author would like to thank his seniors and supervisors for their advice and guidance as well as colleagues who have participated in this audit exercise.
L Lo, MBChB (CUHK), FHKCFP, FRACGP, FHKAM (Fam Med) David VK Chao, MBChB (Liverpool), MFM (Monash), FRCGP, FHKAM (Fam Med) Correspondence to: Dr L Lo, Department of Family Medicine and Primary Health Care, United Christian Hospital, 130 Hip Wo Street, Kwun Tong, Kowloon, Hong Kong, SAR. References
|
|||||||||||