June 2012, Volume 34, No. 2 |
Original Articles
|
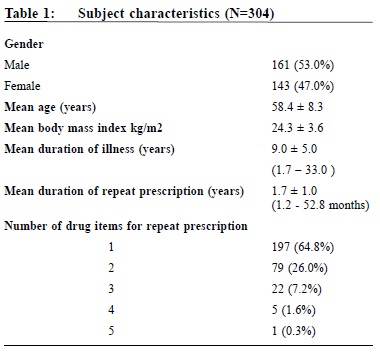
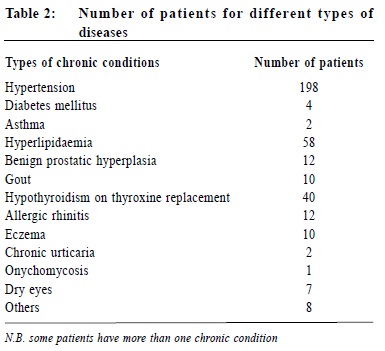
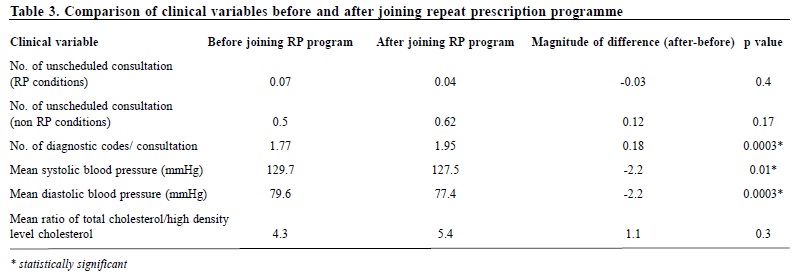
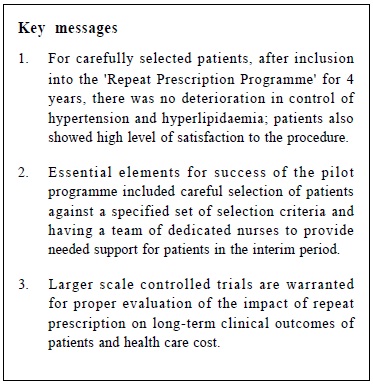
A pilot study on sexually transmitted diseases and high risk sexual behaviour in Hong Kong female ketamine usersTammy KW Tam 譚嘉渭, KH Kwok 郭冠雄, Luke CY Tsang 曾昭義 HK Pract 2012;34:57-63 Summary Objective: To study changes in clinical parameters and pattern of health seeking behavior of patients after joining the ‘repeat prescription’ programme. To report on rate of adverse events and patient’s level of satisfaction. Design: Retrospective cohort. Subjects: Patients who joined the repeat prescription programme in a primary care clinic from May 2004 to February 2008. Main outcome measures: Mean blood pressure and lipid level, number of diagnostic codes per consultation, number of breakthrough consultations. Rate of adverse events. Level of satisfaction. Results: By the end of the study period, 210 subjects who have been in the programme for ≥ 1 year were included for outcome analysis. Significant reductions in mean systolic blood pressure of 2.2 mmHg (p=0.01) and diastolic blood pressure of 2.2 mmHg (p=0.0003) were observed; there were no significant changes in lipid levels (p=0.30). Significantly more diagnostic codes were entered per consultation (p=0.0003). No adverse event was reported. 99% of patients were satisfied or highly satisfied with the programme. Conclusion: This pilot study shows that a repeat prescription arrangement is worthy of development in the context of primary care. Further studies in larger scale, longer duration, more clinical conditions, involving cost effectiveness considerations might be beneficial for the increasingly overloaded public primary health care sector. Keywords: Repeat prescription, chronic illness, clinical outcome 摘要 目的:研究參加重複處方項目的患者臨床指標及求醫行為模式的變化,並報告其不良事件率和患者滿意度。 設計:回顧性隊列研究。 對象:2004年5月至2008年2月間,某基層醫療診所參加重複處方項目的患者。 主要測量內容:血壓和血脂平均值、每次就診的診斷編碼數量、未預約就診的次數、不良事件率和滿意度。 結果:截至研究結束,共有210位加入時間已達到或超過1年,被吸納做臨床結果分析。血壓下降有顯著意義,收縮壓平均值下降2.2mmHg(p=0.01),舒張壓下降2.2mmHg (p=0.0003);血脂水平無顯著變化 (p=0.30);每次就診的診斷編碼數量有所增加 (p=0.0003)。無不良事件報告;99%的患者對此項目感到滿意或十分滿意。 結論:臨床效果預備研究顯示,重復處方項目在基層醫學領域有發展價值,進行更大規模、更長時間、包含更多臨床疾病和成本效益的綜合研究,可能對於日漸超負荷運作的公共醫療服務有所幫助。 主要詞彙:重複處方,慢性疾病,臨床結果 Introduction For most patients with stable chronic medical conditions , their ritualistic return to medical consultations involves mainly confirmation of their stable clinical status and re-stocking of necessary medications. This ebb and flow of patients utilizes a significant amount of healthcare resources. ‘Repeat prescription’ refers to the issuing of a subsequent prescription within a given period, whether it is accompanied by a doctor ’s consultation or not.1 This practice has been widespread in health care for some time. In Western countries, the proportions of repeat prescription range from 29% to 75% of all items prescribed,2-3 a substantial part of which involves medications that are potentially toxic such as psychotropics, cardiac drugs and anti-epileptic drugs, and are issued without direct doctor-patient contact.4-5 There is also a wide variation in reviewing policies between practices; a clinical review published by De Smet & Dautzenberg in 2004 demonstrated that 66% of repeat prescriptions were not periodically reviewed in the previous 2 years.1 The potential benefits of repeat prescription without a doctor’s consultation are reduced costs for the service provider, expanded servicing capacity of the healthcare system and increased convenience for the patient.1 On the other hand, traditional models of repeat prescription have been criticized on its compromised quality on patient care and failure to monitor the effectiveness and safety of the prescribed medication regime.1 No doubt, repeat prescribing in the absence of patient contact should never be undertaken as a light matter. To address the drawbacks of repeat prescription, studies have been conducted, targeting at developing interventions to improve the quality and process of repeat prescriptions.6-9 Nonetheless, published studies have yet to evaluate repeat prescription objectively in terms of its humanistic, economic or clinical outcomes to guide future approach in its utilization. A controlled programme of repeat prescription was piloted in a primary care clinic in May 2004, with an aim to improve the organization and monitoring of the process of repeat prescription, and hoping to achieve an optimal balance between increasing the clinic’s servicing capacity and yet upholding drug safety and good standard of patient care. The pilot stage of this programme recruited only patients with stable chronic diseases. At the end of 2008, the clinic had around 44,000 patients in its registry. For the clinic, all medical appointments were normally made by telephone booking up to 1 week in advance of the requested day of appointment. For patients with chronic illnesses/conditions like hypertension, diabetes mellitus, etc., at each attendance, they would be given a follow-up appointment to enable continued care and monitoring by the attending physician. Usually, the follow-up interval would be 12 weeks and at most 16 weeks, which is also the maximum duration of drug dispensed. We hope that the results generated from the pilot repeat prescription programme would provide more insight to development of future repeat prescription model in primary care setting, and help to improve safety of patients on repeat prescription. Method Our study was conducted in a public funded primary care clinic in Hong Kong. After identifying appropriate patients, clinical parameters of patients before and after utilizing the repeat prescription system were compared, and evaluated to check if the process of repeat prescription would affect disease control adversely or modify the patient’s health seeking behavior. Adverse events were carefully monitored. Patients’ satisfaction to the change was also collected. The Repeat Prescription (RP) Programme From May 2004 through February 2008, patients with stable chronic condition were invited to join the RP programme. Under usual care, a patient would be arranged to see the physician every 12 weeks for follow-up. Under the RP programme patients would be followed up at a longer interval - 24 weekly instead of 12 weekly. If conditions allowed, they would be stocked with 12 weeks medications after each consultation, and between the two consultations, a specially trained and experienced nursing colleague will be responsible to check their condition, check their level of satisfaction and arrange re-stocking of the medications for another 12 weeks. The chronic conditions included in the RP programme were hypertension, diabetes mellitus, hyperlipidaemia, gout, asthma, allergic rhinitis, benign prostatic hyperplasia, dry eyes and onychomycosis. The inclusion criteria were a number of clinical assessments that indicated that the chronic condition was stable. (Appendix I) The criteria were decided by the Clinical Practice Improvement Team of our unit after several team meetings with physicians, nursing colleagues, dispensing colleagues, and clerical colleagues. During the study period, chronic patients attending follow-up consultation were screened by their case physician for eligibility for inclusion into the RP programme. Patients attending with any of the listed conditions and had fulfilled the specified inclusion criteria would be personally invited to participate in the RP programme on a voluntary basis. Should any problem related to their illnesses arise in the RP period, for example deteriorated disease control, unscheduled consultations would be made for them to see their case physician as soon as possible; who would then determine whether or not these patients would continue to be included in the RP programme. After starting the RP programme each subject would be given an identification number for quick retrieval of details including name, Hong Kong identity number, type of clinical conditions and name and duration of medications to be re-stocked. Individual chart review was performed to obtain clinical data. To evaluate the clinical outcomes of subjects before and after starting the RP programme, those who had been on the programme for one year or more were included for data analysis, and specific clinical parameters were used for comparison. For instance, for hypertension, the mean recorded clinic blood pressure over the past year, before the change and then over the ensuing year before the last day of consultation were compared. Similarly, for diabetes mellitus, asthma, gout, and hyperlipidaemia, the mean values of glycated haemoglobin, peak flow rate, number of gouty attacks, and the ratio of total cholesterol (TC) to high density level cholesterol (HDLC) respectively were compared. To evaluate whether the health seeking behavior of subjects had been modified after joining the programme, the frequency of subject attending the clinic was also monitored i.e. more unscheduled consultations, either for concerns about the identified conditions or other acute problems; and whether subjects would present with more complaints during subsequent follow-up consultations, reflected in increased diagnostic codes per consultation. The level of satisfaction with the RP programme and the rate of adverse events were also recorded. Statistical analysis The results were expressed as proportions, means, and standard errors where appropriate. Student t test was performed to compare continuous variables and Pearson chi-square test to compare categorical variables. A two-sided p value of < 0.05 was used to indicate statistical significance. All statistical analyses were performed with the use of SAS software, version 9.1. Results During the study period, 304 patients were recruited to the RP programme. Table 1 showed the characteristics of subjects. Over eighty percent of subjects (255) had one chronic illness, the commonest condition being hypertension (Table 2); 90% (276) required less than three types of medications for drug restock. Of the 304 subjects , 210 had been in the programme for one year or more. Analysis of specific clinical parameters showed that the mean blood pressure of the RP group was 127.5/77.4 mmHg; there was a small but statistically significant reduction of 2 mmHg in mean systolic (p=0.01) and diastolic blood pressure (p=0.0003) after joining the programme; and there was an insignificant increase in the ratio of total cholesterol over high density level cholesterol (TC/HDLC). For other types of chronic illnesses, the number of subjects was not adequate for a valid comparison. There was no significant difference in the number of unscheduled consultations, either for identified RP condition or non RP condition, but the number of diagnostic codes per consultation increased after entering the programme (p=0.0003). (Table 3) During the study period , monitoring of subject’s level of satisfaction was conducted by nursing colleagues during the interim patient review. This showed that 99% of subjects were satisfied or highly satisfied with the programme. No adverse events were reported. The drop-out rate from the programme was 6 . 1%. The reasons for dropping out included patient’s preference, unsatisfactory disease control, non-compliance with medications, side effects of drugs, and ineligibility for joining the RP programme. Discussion Results of our study revealed that after entering the programme, blood pressure levels significantly decreased by around 2mmHg. Although this may not be clinically significant, more importantly, by the end of the study period of almost 4 years, these clinical parameters had remained rather optimal with mean SBP measured 120-130 mmHg, mean DBP measured 70-80 mmHg. These initial results were encouraging. The selection criteria established by our team played a crucial role in maintaining a good standard of care for selected patients on RP. The selection criteria enabled case physicians to detect any disease progression early enough and to act promptly, by diverting patients back to the usual care schedule, and had their condition optimized before re-entering the RP programme again. We also found that RP under the current programme had not changed patient’s health seeking behavior to a great degree. Although there was a small but statistically significant increase in the number of minor physical complaints in subsequent consultations (↑0.18 diagnostic code/consultation), this small numerical difference may not be clinically meaningful. And it was a relief to observe that, there was no increase in total number of unscheduled consultations. Participants seemed not to have become excessively worried about their health status or other health problems after joining the RP programme. In fact, most subjects showed high satisfaction to the RP programme and only very few dropped out (6.1%) during the study period. As the most common condition for drug refill was hypertension, and most subjects in this study had home BP machine for self-monitoring, the home readings might have also helped to assure the participants that their condition was stable. Most importantly, the nurses who were available to assess them in the interim period probably provided much needed support that had helped to alleviate any anxiety. This idea was very similar to nurse-led clinics which has become increasingly popular in the recent decade. A randomized controlled trial involving dermatology practice has showed that nurses, by providing effective patient education and support, can help to free up physician's time, allowing physicians to see more patients.10 Potentially, the RP programme may lead to improved cost effectiveness of the healthcare system by enhancing the role of nurses in patient management. Furthermore, during the study period, we observed low rate of patient non-compliance to medications (1.2%) and no adverse event arose that was related to the RP programme. All subjects in the RP programme were given a hotline telephone number through which they could talk with the nurses directly during office hours whenever they encountered problems with the use of mediciations. The high rate of compliance was incontrast with other studies, which reported that 12.4% of patents had compliance problems, side-effects, adverse drug reactions, or drug interactions. 11 Limitations However, at this point, the available evidence is not sufficiently strong for us to conclude that RP under the monitored programme would have no adverse effect on the long term clinical outcomes such as disease complications and mortality as compared with usual care. Obviously, more clinical data are needed and further controlled trials with parallel comparison of the control (usual care system) and intervention (RP system) groups are required to provide a stronger conclusion for this question. Compared with the traditional system of repeat prescription, where a wide variety of medications including antibiotics, psychotropics, anti-epileptics and cardiovascular drugs would be refilled without consultation and for even longer periods, the scale of repeats in our programme was much smaller. One might challenge that the impact of our repeat system on the overall healthcare capacity would be small. However, this was only the pilot stage of our programme. In the era of an aging population, and substantial rise in the prevalence of chronic illnesses, the scale of the RP programme would also expand accordingly. We might also consider extending the period of repeat, and simplifying and streamlining the repeat procedure to promote a higher rate of participation of both doctors and patients. Most importantly, the clinical outcomes of patients should be reviewed regularly. By following the stringent selection criteria, our programme will help to maintain a standard quality of drug use and patient care, which will in turn contribute to a good standard of chronic care modeling. To the best of our knowledge, this study is the first of its kind to objectively evaluate clinical outcomes after repeat prescription. At this pilot stage, the sample size was relatively small compared with the whole clinic population. Time constraint in a busy clinic setting and the extra work involved had been the major barriers for doctors to do the invitations to include patients to the RP programme. With the present sample size, we were only able to assess the blood pressure and serum lipid levels, while comparative analysis for other diseases was not performed. But with the encouraging results obtained in this first phase of programme, we believe that comprehensive evaluation of the whole spectrum of chronic diseases would be possible with further recruitment of patients in the near future. This is a retrospective study to review clinical outcomes of RP patients by the fourth year of the programme. Duration of chronic illness may also affect disease progression, and may become confounders to our results. Standardization of patient’s duration of illness, or conducting clinical trials to compare outcomes between RP group and usual care group would help to reduce the biases involved. As repeat prescription used in long – term pharmacotherapy is often associated with patient nonadherence, 12 under our current system, we were only able to monitor drug compliance for patients who turned up for follow-up, and not for those who defaulted in the interim period. The overall rate of drug non-adherence and its ultimate effect on disease outcomes might be under-estimated. Establishment of a call-back system might be considered to help identify defaulters in the interim period and arrange them for further follow-up. Conclusions After implementation of the RP programme, control of hypertension and hyperlipidaemia remained stable. No adverse events were reported and patients’ satisfaction levels were high in this cohort. The essential elements of success for this pilot programme included careful selection of patients against the specified list of inclusion criteria and having a team of dedicated nurses to provide the needed support in patient review and use of medications. Hopefully, our study has painted a primitive framework for future development of the repeat prescription arrangement, to maximize patient coverage while balancing the quality of patient care and medication safety. Acknowledgement We wish to express our sincere thanks to all doctors and nurses in the Quality Assurance Team of our unit – Dr Tsui Kwok Biu, Dr Fan Yuen Man, Cecilia, Dr Ng Kwok Keung, Dr Ho King Yip, Anthony, Ms Chung Lai Ngo, Joan, Ms Kwong So Ying, Ms Edborough Lai, Ms So Wai Yi, Libby, and Ms So Yuet Ming - for their dedicated work and contribution to the establishment of xthe Repeat Prescription Programme; and also Mr Lau Cheuk Man for his tremendous statistical support for this project.
Tammy KW Tam, MBChB (CUHK), MMedSc (HKU), FRACGP, FHKAM (Fam Med) Family Physician KH Kwok, MBBS (HKU), FHKCFP, FRACGP, FHKAM (Fam Med) Family Physician Luke CY Tsang, MBBS (UNSW), MPH (HKU), FRACGP, FHKAM (Fam Med) Family Physician Correspondence to: Dr Tammy KW Tam, Kowloon Families Clinic, 6/F Yaumatei Polyclinic, 145 Battery Street, Yaumatei, Kowloon, Hong Kong SAR. References
|
|