|
November 2007, Volume 29, No. 11
|
Update Article
|
Chemical pathology case conference - laboratory tests for diabetes mellitusYuet-ping Yuen 袁月冰, Chloe M Mak 麥苗, Angel O K Chan 陳安琪, Michael H M Chan 陳浩明, Rossa W K Chiu 趙慧君, Ching-wan Lam 林青雲, Tony W L Mak 麥永禮, Wing-tat Poon 潘永達, Anthony C C Shek 石志忠, Morris H L Tai 戴學良, Sidney Tam 譚志輝, Albert Y W Chan 陳恩和 HK Pract 2007;29:419-426 Summary Diabetes mellitus is a chronic disease with increasing prevalence in most places in the world. A number of laboratory tests, such as glucose, haemoglobin A1C, urine albumin, creatinine, lipid profile play pivotal roles in diabetes care. Other adjunctive laboratory tests include urine and blood ketone, fructosamine, and C-peptide. Judicious use of these tests is important to ensure all patients are diagnosed and managed according to evidence-based clinical guidelines. Some knowledge about the pre-analytical and analytical aspects and potential pitfalls of these laboratory tests also assist clinicians to interpret test results properly. 摘要
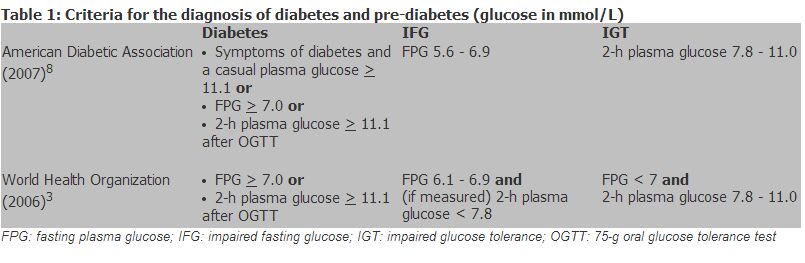
糖尿病是一種慢性疾病,在世界大多數地區,它的病發率都在上升。血糖、血紅蛋白A1C、尿蛋白、肌酐、 血脂等化驗檢查在治療糖尿病時扮演著重要的角色。其他輔助化驗項目還有尿酮體和血酮體、果糖胺和C Introduction Diabetes mellitus is a chronic disease with increasing prevalence in most places in the world. In Hong Kong, it is estimated that 1 in 10 people have diabetes.1 There are a number of national and international clinical guidelines for diabetes mellitus. In particular, the American Diabetes Association (ADA) and the World Health Organization (WHO) guidelines are widely adopted by health care providers globally, including Hong Kong. Table 1 lists the diagnostic criteria of diabetes and pre-diabetes from ADA and WHO. The Diabetes Division, Hong Kong Society for Endocrinology, Metabolism and Reproduction also published a statement on the clinical diagnosis and management for type 2 diabetes mellitus in year 2000.2 To implement any one of these guidelines in clinical practice, the use of multiple laboratory tests is necessary. This article focuses on the pre-analytical and analytical aspects of laboratory tests that are used in diagnosis and management of diabetes. Readers can refer to the published clinical guidelines for other aspects of diabetes care like classification, management and treatment targets.
Laboratory tests for diagnosis The diagnosis of diabetes depends on the measurement of fasting plasma glucose (FPG) or the 2-hour plasma glucose after an oral glucose tolerance test (OGTT).3,4 A random plasma glucose level can also be used for diagnosis if patients have symptoms like polydipsia, polyuria and weight loss. Other tests like glycated haemoglobin (GHb) is currently not recommended for this purpose.3,4 Before taking a blood sample for FPG, patients have to be fasted for at least eight hours.4 Fasting plasma glucose has a diurnal variation, with mean level higher in the morning than in the afternoon.5 Therefore, performing the test in the afternoon may miss some patients with diabetes.6 Glucose concentrations in blood samples decline continuously after blood collection because of glycolysis. Antiglycolytics like sodium fluoride (NaF) is commonly used to minimize this ex vivo change. If cells can be separated within an hour after blood collection, antiglycolytics are not required and heparin plasma can be used. Glucose can be measured in plasma and whole blood collected from different sites (venous, capillary and arterial). Glucose concentration in plasma is approximately 11% higher than that of whole blood because of higher water concentration in plasma. However, this difference in glucose concentration would vary with changes in the haematocrit level.7 Because of this correlation problem between plasma and whole blood glucose, both ADA and WHO recommend the use of venous plasma glucose for diagnosis of diabetes. All glucose measurements used for diagnosis of diabetes have to be performed in a clinical laboratory using a laboratory analytical method. Glucometers, which has limited accuracy and precision at both high and low glucose concentrations, is not suitable. Because of the relatively large intra-individual biological variations, repeat testing is required to confirm a diagnosis. Laboratory tests for determining the aetiology of diabetes
Immune-mediated type 1 diabetes is caused by autoimmune destruction of pancreatic
Most patients are classified as type 1 or type 2 diabetes on the basis of age of
disease onset, mode of presentation, insulin dependence, family history and body
mass index. Specific diagnostic tests like autoimmune markers are rarely necessary.6
C-peptide is used as a marker of
Hormones including cortisol, growth hormones, glucagon and adrenaline antagonize the actions of insulin. Therefore, some endocrine diseases like Cushing syndrome, acromegaly, glucagonoma and phaeochromocytoma can cause secondary hyperglycaemia. Both hyperthyroidism and hypothyroidism affect diabetes management. Hyperthyroidism can worsen glycaemic control and even precipitate diabetic ketoacidosis (DKA). On the other hand, patients with hypothyroidism are at increased risk of hypoglycaemia and more severe dyslipidaemia. Signs and symptoms of these endocrine diseases should be looked for in newly diagnosed diabetic patients. If there is any clinical suspicion, specific laboratory investigations should be performed. Mitochondrial DNA mutations like 3243A>G can cause maternally inherited diabetes. High incidence of deafness is found in this group of patients.12 Genetic tests for hotspot mutations in mitochondrial DNA (mtDNA) are now available as routine tests in some local clinical laboratories. Patient with clinical features of mitochondrial disorders (e.g. maternal inheritance, deafness, short stature, myopathy and any neurological complaints) should be referred for genetic testing.13 Another genetic form of diabetes is maturity onset diabetes of the young (MODY). At least eight different types of MODY have been characterized at the molecular level and all of them are inherited in an autosomal dominant manner. Genetic tests for MODY is still largely performed in research settings and no routine service is available in local laboratories. Laboratory tests for complication detection Proteinuria Diabetic nephropathy is one of the most common causes of end-stage renal disease and its first manifestation is increased urine albumin excretion.14 Albuminuria of diabetic nephropathy is divided into two stages - microalbuminuria and macroalbuminuria (Table 2). The use of 24-hour or timed urine collection is the traditional way for diagnosis of microalbuminuria. However, spot urine sample is now recommended for urine albumin measurement and the result is expressed as albumin-to-creatinine ratio (ACR) (units: mg/mmol creatinine or mg/g creatinine) or simply as albumin concentration (mg/L).14-16 The use of spot urine is convenient to patients and it also eliminates collection error that is common in 24-hour urine collection. Different cut-offs of ACR are used to define micro- and macro-albuminuria in different clinical guidelines.14,16 The cut-off values that are commonly used by local clinicians are listed in Table 2. The use of creatinine in ACR is to correct for variations caused by hydration. However, urine creatinine concentration is determined by factors like age, sex and muscle mass. Therefore, some advocate the use of sex-specific ACR cut-offs (> 2.5 mg/mmol creatinine for males and > 3.5 mg/mmol creatinine for females) as female subjects have lower urine creatinine concentration than male subjects.16,17
First morning urine is the preferred sample for ACR measurement but random urine sample is also acceptable.14,16 However, significant discrepancy between ACR from first morning urine and random spot urine has been reported.18 Therefore, consistent time of urine collection may make serial ACR results of a patient more comparable. Interpretation of results may not be straight-forward when in the presence of the following conditions that may increase urine albumin excretion: urinary tract infection, haematuria, acute febrile illness, vigorous exercise, short-term pronounced hyperglycaemia, uncontrolled hypertension and congestive heart failure.14 Most clinical laboratories measure urine albumin by commercial assays using either immunoturbidimetry or immunonephelometry. The detection limits and precision of these methods allow the accurate measurement of urine albumin at low concentrations.6 Different dipsticks are available for measurement of urine proteins. Standard urine dipsticks (e.g. Albustix) detects total protein in urine using pH indicator dye which changes colour upon binding to negatively charged proteins. These dipsticks are not sensitive enough for detection of microalbuminuria. More sensitive dipsticks for qualitative or semiquantitative measurements of microalbuminuria are also available. These microalbumin dipsticks have acceptable sensitivity and specificity for detection of albumin concentration at 20 mg/L, a cut-off used in some national guidelines for microalbuminuria.16 However, their performance is inferior to ACR measured by laboratory methods.19,20 So when laboratory tests for microalbumin are not readily available, microalbumin dipsticks can be used. However, all positive results have to be confirmed by a laboratory test. All type 2 diabetic patients should be screened for diabetic nephropathy at initial assessment. For type 1 diabetic patients, screening may start five years after disease onset.14 A spot urine sample should be first checked for the presence of protein using standard urine dipstick. If it is negative, a test for either ACR (spot urine) or urine albumin excretion rate (UAER, 24-hour or timed urine) should follow. Because of the large intra-individual biological variation, repeated measurements of ACR or UAER are required to confirm the diagnosis.14 On the other hand, if a spot urine is tested positive for protein by standard urine dipstick, overt nephropathy is present. Then urine total protein should be measured. Renal function and glomerular filtration rate (GFR) In diabetic patients, assessment of glomerular function is as important as albuminuria screening. Significant decline in GFR has been noted in patients with type 1 and type 2 diabetes and normal urine albumin excretion.14 Therefore, screening for increased urine albumin alone would miss a considerable number of diabetic patients with chronic kidney disease. However, plasma creatinine level is determined by many factors other than GFR and it is not sensitive for detection of mild decrease in GFR. Other methods of GFR assessment for routine clinical use include calculation of creatinine clearance (CrCl) using 24-hour urine samples and the use of estimation equations based on plasma creatinine and other parameters. More detailed discussions about the merits and problems of different GFR assessment methods can be found in a previous Chemical Pathology Case Conference.21 Lipid profile Elevated triglyceride with low HDL-cholesterol is commonly seen in diabetic patients. As both diabetes and hyperlipidaemia are risk factors for cardiovascular diseases, all newly diagnosed diabetic patients should have a fasting lipid profile checked. Severe hypertriglyceridaemia (>30 mmol/L) can be seen in patients with recent onset diabetes. Blood samples from these patients appear turbid to naked eyes. Laboratory tests for monitoring of glycaemic control Glucose Self-monitoring of blood glucose (SMBG) using glucometers plays an important role in routine monitoring of diabetes. As mentioned above, glucose concentrations in plasma and whole blood are different. Some glucometers measure glucose concentrations in whole blood sample and are programmed to report plasma glucose concentrations but some glucometers still report whole blood glucose values.3,22 Therefore, it is important to check the package inserts of glucometers. Glucose concentrations in capillary and venous whole blood are similar in fasting samples. However, a difference of 20 - 25% (capillary higher than venous) can be seen in postprandial samples.23 Glucometer readings are not only used to monitor daily glycaemic control, they also guide the dosages of insulin and help detect hypoglycemia. Proper quality control of glucometer is thus crucial. The newer models of glucometers are more user-friendly and require less skill to perform a test. Nonetheless, patients should be instructed the correct use of glucometers regularly. Calibration and quality control should be performed according to the manufacturer"s instructions. Periodic parallel test with laboratory glucose analysis will help monitor the performance of the glucometers. Moreover, test strips should be stored properly and all expired test strips should not be used. Faulty strips may give erroneous results that lead to serious consequences. Glucometers are usually calibrated to cater for a normal haematocrit of 40 - 50%. They give lower glucose results when the haematocrit is above this range and higher glucose results when the haematocrit is below this range.24 However, this would not be a significant problem in most diabetic patients as their haematocrit levels would not deviate much from the normal range. Likewise, other factors that have been reported to influence glucometers readings (e.g. partial pressure of oxygen in blood, the presence of sugars like maltose in blood, etc.) have little impact on ordinary diabetic patients.25,26 Urine glucose testing, once commonly used in home glucose monitoring, has been replaced by SMBG.23 Urine glucose testing is easy to perform and convenient to patients, but its clinical usefulness is limited as it provides no information about blood glucose levels below the variable renal threshold (~ 10 mmol/L). It reflects only the average glucose values between voiding and a normal result cannot distinguish euglycaemia from hypoglycaemia. Together with other deficiencies like poor performance at low glucose levels, urine glucose testing should only be relied upon when there is no better alternative.23 Glycated haemoglobin (GHb) Glycated haemoglobins (GHb) are formed by non-enzymatic reactions between glucose and the amino acid residues of haemoglobin (Hb) molecules, and its level is dependent on the blood glucose concentration. Therefore, GHb level is used as an index of average glycaemia in the previous 2 - 3 months (i.e. the average lifespan of red blood cells).23 Haemlglobin A1C (Hb A1C) is one of the many species of GHb exist in the circulation. It is a specific GHb with a glucose attached to the NH2-terminal valine of one or both b-chains. GHb is the standard for monitoring glycaemic control in diabetic patients because its concentration in blood is linked to the risks of development of diabetic complications as shown by the Diabetic Control and Complications Trials (DCCT) and the United Kingdom Prospective Diabetes Studies (UKPDS).27,28 To adopt the results of these prospective studies, HbA1C have to be measured by assays that are certified to produce DCCT-aligned values. More information about the certified HbA1C methods can be found in the National Glycohemoglobin Standardization Program (NGSP) website (www.ngsp.org). A consensus statement on the worldwide standardization of HbA1C measurement was published recently, which suggested standardizing of all HbA1C assays to the International Federation of Clinical Chemistry (IFCC) reference system and reporting HbA1C results in mmol/mol in addition to the traditional unit (%).29 It is expected that most of the HbA1C assay manufacturers will follow this new consensus guideline and a change in the HbA1C assays will occur in the near future. All diseases and conditions that affect red blood cell (RBC) survival or alter the average age of circulating RBC affect HbA1C results irrespective of the analytical methods used. For example, haemolytic anaemias decrease HbA1C as RBC survival is shortened. Apart from this, HbA1C measurements are also affected by Hb variants (e.g. HbS, HbC and HbF) and Hb derivatives (e.g. carbamylated Hb which is elevated in patients with uraemia) in ways that are independent on their effects on RBC survival.30 Moreover these effects are method dependent. A summary of reported interferences of different Hb variants on different HbA1C assays can be found in the NGSP web site. Hemoglobin F is the fetal hemoglobin with two a and two g chains. The level of HbF usually falls to the adult level (< 1%) at two years of age.31 The effects of HbF on HbA1C assays are variable and most of the reported HbF interference occurs when HbF is higher than 10%.32 The most common haemoglobinopathies among Chinese are thalassemias. It has been demonstrated that most commercial methods could measure HbA1C correctly in beta-thalassemia trait samples.33-35 Iron status also affect the interpretation of HbA1C results. It has been shown that in both diabetic and non-diabetic subjects with co-existing iron deficiency, HbA1C levels dropped significantly after iron replacement.36 Fructosamine Fructosamine, a collective term for serum glycated proteins, is less commonly used than HbA1C in routine monitoring of glycaemic control. Serum proteins (mainly albumin) have a circulating half-life of around 20 days and therefore fructosamine can be used as an index of glycaemia over a period of 2 - 3 weeks. Assays for fructosamine are not widely available in local clinical laboratories, probably related to the small clinical demand. Fructosamine may be used in patients whose HbA1C levels do not reliably reflect glycaemic control (e.g. patients with haemolytic anaemias). Fructosamine levels are affected by any diseases and conditions that affect the half-life of plasma proteins, such as nephrotic syndrome. The values are also unreliable when there are gross changes or rapid changes in plasma protein concentrations.6 Urine and blood ketones
As recommended by ADA, urine ketone testing can be used in routine diabetic monitoring
especially for patients with type 1 diabetes and pregnant diabetic patients.23
All urine ketone testing devices detect acetoacetate, and some also detect acetone.
However, these devices cannot measure
Conclusion Quality care of diabetic patients depend heavily on use of laboratory tests, either performed in a clinical laboratory, at the site of patient care or by patients themselves as home monitoring. FPG and OGTT are the only tests for diagnosis of diabetes. All glucose measurements for diagnosis should be measured by a laboratory analytical method and not by glucometers. Specific diagnostic tests like autoimmune markers and C-peptide for differentiating type 1 and type 2 diabetes are not necessary in the majority of patients. With increasing knowledge of the underlying genetic causes of diabetes and more readily assessable testing facilities, selected patients should be referred for genetic testing. Certain endocrinopathies can cause secondary hyperglycaemia or worsen glycaemic control. Appropriate endocrine tests should be performed if there is a clinical suspicion. Regular screening for microalbuminuria using either ACR or UAER is indicated for all diabetic patients. Dipstick for microalbuminuria can be used when laboratory tests for microalbumin is not readily available but any positive results have to be confirmed by a laboratory test. Glomerular functions of diabetic patients may deteriorate without increase in urine albumin excretion. Therefore, regular assessment of GFR is necessary. All diabetic patients should have lipid profile checked regularly. Diabetes per se can lead to dyslipidaemia and both diabetes and dyslipidaemia contribute to increased risk to cardiovascular diseases. Glucometers are commonly used at the site of patient care and as home monitoring. As the tests are performed outside laboratories, it is the responsibility of the operators to perform regular calibration, quality control and maintenance according to the manufacturer"s recommendations. A good quality control system should be established to ensure accurate results from glucometers. Haemoglobin A1C plays an indispensible role in monitoring glycaemic control. Global standardization would improve the comparability of test results from different laboratories. However, standardization alone cannot solve some of the inherent problems of HbA1C assays like interference by Hb variants and Hb derivatives. Key messages
Yuet-ping Yuen, MBChB(CUHK), FRCPA, FHKAM(Pathology)
Resident Specialist, Albert Y W Chan, MBChB(Glasg), MD(CUHK), FHKCP, FHKCPath Consultant Chemical Pathologist, Department of Pathology, Princess Margaret Hospital. Chloe M Mak, MBBS, FRCPA, FHKCPath, FHKAM(Pathology) Resident Specialist, Sidney Tam, FRCP(Edin), FRCPA, FHKAM(Medicine), FHKAM(Pathology) Head and Consultant, Division of Clinical Biochemistry, Department of Pathology, Queen Mary Hospital. Wing-tat Poon, MBChB(CUHK), MRCP, FHKCPath, FHKAM(Pathology) Resident Specialist, Tony WL Mak, MBChB(CUHK), MBA, FRCPath, FRCPA, FHKAM(Pathology) Consultant, Hospital Authority Toxicology Reference Laboratory. Angel OK Chan, BMedSc(Hons,CUHK), MBChB(CUHK), FRCPA, FHKAM(Pathology) Resident Specialist, Anthony CC Shek, MBBS(HK), FRCPath, FRCPA, FHKAM(Pathology) Consultant, Department of Pathology, Queen Elizabeth Hospital. Michael HM Chan, MBChB(CUHK), FRCPA, FHKCPath, FHKAM(Pathology) Associate Consultant, Rossa WK Chiu, MBChB(Queensland), PhD(CUHK), FRCPA, FHKAM(Pathology) Professor, Ching-wan Lam, MBChB(CUHK), PhD(CUHK), FRCPA, FHKAM(Pathology) Associate Professor, Morris HL Tai, MBChB(CUHK), FRCPA, FHKAM(Pathology) Medical Officer, Department of Chemical Pathology, Prince of Wales Hospital, Chinese University of Hong Kong. Correspondence to : Dr Yuet-ping Yuen, Department of Pathology, Princess Margaret Hospital, Kowloon, Hong Kong.
References
|
|