Summary
Rheumatoid arthritis and ankylosing spondylitis are chronic diseases that frequently lead to disability and social disadvantages. Recent advances in biologic therapy has made unprecedented disease control, retardation of radiographic progression and improvement in functional outcomes possible, both in refractory and early diseases. Clinicians should bear in mind the increased risks of infection especially tuberculosis, heart failure and demyelinating disease when considering the use of anti-TNF 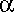 .
.
摘要
類風濕性關節炎和強直性脊椎炎是經常引致傷殘和社交障礙的慢性疾病。生物治療的最新發展適用於早期和病情頑固的病人,可以控制病情,延緩X光改變並且改善功能。醫生應注意anti-TNF 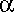 (抗
(抗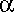 腫瘤壞死因素藥物),會增加感染,特別是肺結核、心臟衰竭和脫髓鞘病的風險。
腫瘤壞死因素藥物),會增加感染,特別是肺結核、心臟衰竭和脫髓鞘病的風險。
Introduction
Rheumatoid arthritis (RA) is a chronic disease characterized by a symmetrical polyarthritis, which is commonly followed by joint deformities and disability in the long run.
It has been recognized that 75% of patients with RA will have joint erosions within the first two years of disease,1 which herald the development of joint deformities. With the accumulation of joint deformities, disability also accumulates. In fact, researches have found that only 40% - 60% of RA patients would remain in employment 5 years after disease onset.2 After 20 years, 60% of the patient would become functionally dependent. In contrast to the common concept that RA is a benign disease, patients with poorly controlled RA would have a mortality rate comparable to triple-vessel ischaemic heart disease or stage III/IV Hodgkin's lymphoma.3 In fact, premature mortality in RA patients is recognized in recent years, in which the standard mortality ratio of RA being 1.22 to 1.89, and is the highest among patients with more severe disease.4,5
The management of rheumatoid arthritis involves drug and non-drug therapies. The weight of the disease has put forward the importance of early treatment of RA instead of the old concept of a pyramidal approach, in which disease modifying anti-rheumatoid drugs (DMARDs) were used only as the last resort. Methotrexate (MTX) is currently the anchor DMARD, used either as a monotherapy or in combination with other DMARDs. Advances in the management of RA must include the development of biologic therapies which would allow an unprecedented disease control.
Ankylosing spondylitis (AS) is characterized by chronic, progressive inflammatory back pain and enthesitis affecting about 0.2% of the population among Chinese.6 Males are affected more frequently with symptoms usually starting in their late teens or early 20's. Progressive stiffness of the spine, leading to kyphosis and "reversed question mark" posture is not uncommonly seen. AS also carries much social impact. In 5 years, 13% of the patients would become unemployed and the rate rose up to 31% in 20 years.7 Apart from non-steroidal anti-inflammatory drugs (NSAIDs) and in some cases, sulphasalazine, there has been few medical treatment for AS. Not until recent years did the use of biologic therapy revolutionize the management of AS.
Biologic therapy for rheumatoid arthritis
A number of biological therapies for RA is made possible due to the recent advances seen in the field of biotechnology. These include: anti-tumour necrosis factor-a (TNFa) agents, interleukin(IL)-1 receptor antagonist, anti-IL-6 receptor antibody, anti-IL-15 antibody, cytotoxic T-lymphocyte antigen-4 immunoglobulin, anti-CD20 antibody, etc. This article will focus on the most commonly available biologic agents, anti-TNFa therapies.
Chronic inflammation and pannus formation is the hallmark in the pathogenesis of RA. Signaling the activation of inflammatory cells is largely an act of IL-1 and TNFa, which are potent stimulators of synovial fibroblasts, osteoclasts, and chondrocytes, which in turn releases tissue-destroying matrix metalloproteinases.8 Targeted inhibition of TNFa can therefore effectively inhibit the inflammatory process and leads to disease control.
There are currently 3 anti-TNFa agents available. Infliximab and adalimumab are monoclonal antibodies against TNFa, in which the former is a chimeric antibody (hybrid of human and mouse sequences) while the latter is a fully humanized one. Etanercept, on the other hand, is a fusion protein of soluble TNFa receptor and immunoglobulin. There are many clinical trials demonstrating the efficacy of these agents in the treatment of RA. Some of the landmark studies will be discussed here.
Anti-Tumour Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy (ATTRACT) study9,10
The ATTRACT study is a randomized, double-blind, placebo-controlled trial in which 428 patients with active, established RA despite methotrexate therapy were divided into 5 groups receiving 3mg/kg or 10mg/kg of the infliximab, at 4- or 8- week intervals for 1 year, along with MTX, versus MTX alone. Greater efficacy of infliximab at various doses were noted, represented by a higher proportion of patients achieving ACR20, ACR50 and ACR70* (42-59% vs. 17%, 21-39% vs. 8%, 10-25% vs. 2%, respectively, p<0.001 for all comparisons except for infliximab 3mg/kg every 8 weeks vs. MTX where p=0.04) which represent 20%, 50% and 70% improvement, respectively. Apart from clinical improvement, inhibition of radiographic progression was noted in patients receiving infliximab, and the inhibition was sustainable up to 102 weeks in an extension of the study. Functional improvement was also demonstrated in the study.
Trial of Etanercept and Methotrexate with Radiographic Patient Outcomes (TEMPO) study11
The TEMPO study is a study on the use of etanercept, versus MTX, or the combination of the two in patients with established RA who failed at least one DMARD but who had not been recently exposed to MTX. Altogether there were 682 patients randomly allocated into one of the three groups. At 1 year, higher proportions of patients in the group receiving both etanercept and MTX achieved ACR20, ACR50 and ACR 70, compared with patients on either drug alone (85% vs. 75-76%, 69% vs. 43-48%, 43% vs. 19-24%, respectively, p<0.05). Greater inhibition of radiographic progression was also achieved by the combination when compared with etanercept monotherapy, which in turn was more efficacious than MTX monotherapy. Combination of etanercept and MTX also led to a greater functional improvement. This study demonstrated that a combination of MTX and anti-TNFa therapy is superior to either drug used alone.
Etanercept in Early RA (ERA) study12
The ERA study recruited 632 patients with early RA (disease 3years) and randomized them into two groups which received etanercept (10 or 25 mg) twice weekly or MTX once weekly (mean dosage 19 mg per week) for 1 year. The percentages of patients receiving 25 mg of etanercept who managed to achieve ACR20, ACR50, and ACR70 were significantly greater than those in the MTX group at most evaluations within the first six months. However, thereafter the results were approximately the same.
* ACR20, ACR50 and ACR70 represent 20%, 50% and 70% improvement in clinical parameters including tender joint counts, swollen joint counts, erythrocyte sedimentation rate or C-reactive protein, patient's global assessment, physician's global assessment and general health.
At 12 months, the proportion of patients achieving ACR20 were not significantly different (72% vs. 65%, p=0.16). Despite similar clinical efficacy, patients on etanercept had significantly less radiographic progression than patients on MTX at 2 years.
The Anti-Tumor Necrosis Factor Research Study Program of the Monoclonal Antibody Adalimumab in Rheumatoid Arthritis (ARMADA) study13
In the ARMADA study, 271 patients with active RA despite treatment with MTX were randomized into groups receiving MTX plus adalimumab at 20, 40, 80 mg once every 2 weeks or MTX alone. At 24 weeks, ACR20 were more often met by patients on MTX plus adalamumab at 20 mg, 40 mg and 80 mg, than those on MTX alone (47.8%, 67.2%, 65.8%, vs. 14.5%, p<0.001). Similarly, ACR50 and ACR70 were achieved by a greater proportion of patients on different doses of adalimumab. An extension of the study showed a significant inhibition of radiographic progression for the adalimumab groups compared with methotrexate group at 52 weeks.
Biologic therapy for ankylosing spondylitis
Similar to RA, TNFa is also a key cytokine underlying the active inflammation of the spine. In biopsies of the sacro-iliac joints in early AS, dense mononuclear infiltrates and TNF mRNA were detected. Therefore anti-TNFa therapy can effectively control the spinal inflammation in AS.
Infliximab in AS Study by Braun et al14
Braun et al randomized 70 patients with active AS, average disease duration of 15 years into groups receiving either 5mg/kg of infliximab at 0, 2, 6 weeks or placebo. He demonstrated that significantly more patients on infliximab achieved a 50% improvement as measured by the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) (53% vs. 9%, p<0.05) and the effect was sustained up to 12 weeks. Similarly, 20% and 70% improvement were more often seen in patients on infliximab. Patients on placebo who switched over to infliximab after 12 weeks also showed satisfactory response which lasted up to 102 weeks in an extension of the study.15
ASSERT Study16
A larger study by van der Heijde et al involved 279 patients who were randomized into infliximab or placebo given at 0, 2, 6, 12 and 18 weeks. Assessment was done for up to 18 weeks and the Assessment in Ankylosing Spondylitis 20% improvement (ASAS20) was achieved by 61.2% of patients on infliximab vs. 19.2% of patients on placebo (p<0.001). Besides functional improvements, some structural improvements were also seen.
Etanercept in AS Study by Davis et al17
In this study, 277 patients with active AS were randomized into etanercept 25 mg twice weekly or placebo for 24 weeks. ASAS20 response was achieved by 59% of patients on etanercept vs. 28% of patients on placebo (p<0.0001). An extension of the study in which patients on placebo switched to etanercept showed that the drug was effective up to 96 weeks.
The results of the above studies are summarized in Table 1 and Table 2.
Side effects of anti-TNFa therapy
Post-marketing surveillance noted that anti-TNFa agents were associated with a greater risk of infection. A report published in 200118 revealed that out of 147,000 patients exposed to infliximab worldwide by May 2001, 70 cases of tuberculosis were identified. The cases of tuberculosis developed at a median of 12 weeks post infliximab treatment, 40 out of the 70 cases were of the extra-pulmonary tuberculosis type. Apart from tuberculosis, cases of opportunistic infections such as listeriosis, histoplasmosis, aspergillosis, severe candida infections were also reported.
Later cases of tuberculosis and other opportunistic infection were also reported in patients receiving etanercept and adalimumab, although the incidence appeared lower. Since these reports, more careful selection of patients by careful history, chest radiograph, Mantoux test or Heaf test, and the use of isoniazid prophylaxis, resulted in reduced incidence of tuberculosis among patients receiving anti-TNFa agents.
Apart from infection, demyelinating disease, aplastic anaemia, thrombocytopenia, agranulocytosis, new development or worsening of congestive heart failure were also noted in patients receiving anti-TNFa therapy. Although suggested by its name, the incidence of tumour, e.g. lymphoma, was not significantly raised in patients receiving the drug when compared with other RA patients.
British Society of Rheumatology (BSR) Guidelines on the use of Anti-TNFa agents in RA and AS
In view of the side effects of the anti-TNFa agents, and their costs, the BSR issued guidelines on the use of anti-TNFa agents in RA and AS (Box 1 and 2). In short, patients must have active disease despite DMARDs, or NSAIDs in case of AS, and have no contraindication to the anti-TNFa agents. Contra-indications to anti-TNFa therapy include mulitple sclerosis, active infection or high risk of infection, recent septic arthritis, infected joint prosthesis, congestive heart failure, and history of cancer. Those who have history of tuberculosis should be treated first if the previous treatment was judged to be inadequate. Those who have had adequate treatment before or who have had latent tuberculosis may be offered prophylaxis before the start of therapy. Patients' responses to therapy have to be monitored regularly and those who fail to respond in a defined period of time should either be withdrawn or switched to another anti-TNFa agent or other biologic agents.
Consensus statements on the indications and monitoring of anti-TNF therapy for rheumatic diseases in Hong Kong
In view of the rapid emergence of TNF antagonists, The Hong Kong Society of Rheumatology had established a local consensus on the use of the anti-TNF agents in co-operation with local rheumatologists, chest physicians and other relevant specialists. The consensus was made with special attention to the endemicity of tuberculosis and the high prevalence of Hepatitis B in Hong Kong. With regard to tuberculosis, a detailed pre-treatment evaluation including history, chest radiograph, Mantoux test are advised. Latent tuberculosis, as defined as a positive Mantoux test but no sign and symptom of active tuberculous infection, should be treated with isoniazid for at least 9 months, and initiation of anti-TNF therapy is advisable only after one month of isoniazid treatment. For those who have a history of extensive or life-threatening pulmonary or extra-pulmonary tuberculosis, anti-TNF therapy is best avoided.
The consensus statement is available at the web site of the Hong Kong Society of Rheumatology: http://www.fmshk.com.hk/hksr/.
Conclusion
Rheumatoid arthritis and ankylosing spondylitis pose significant morbidity and the social impact of the two diseases is huge. Revolutions in the management of RA and AS have led to earlier treatment and more effective therapy. The development of biologic therapy, e.g. anti-TNFa therapy, has started a new era in the
Declaration of interests : none
Key messages
- Rheumatoid arthritis (RA) and ankylosing spondylitis (AS) are associated with poor clinical and functional outcomes.
- Recent development of biologic therapy revolutionized the management of RA and AS.
- Many clinical trials have demonstrated the superior efficacy of anti-TNFa therapies in RA and AS over conventional treatment.
- Infection, in particular tuberculosis, demyelinating disease, aplastic anaemia, thrombocytopenia, congestive heart failure are reported side effects of anti-TNFa agents.
- Careful selection of patients and close monitoring of side effects are necessary when using anti-TNFa therapies. management of RA and AS. Careful selection of patients should be exercised with potential side effects being closely monitored.
Lucia S Y Chau, MBBS, MRCP, FHKCP, FHKAM (Medicine)
Resident (Specialist),
Alexander M H Leung, MBBS, MSc Rheu (Birm), FHKCP, FHKAM (Medicine)A
ssociate Consultant,
Department of Medicine, Queen Elizabeth Hospital.
Correspondence to: Dr Lucia S Y Chau, Department of Medicine, Queen Elizabeth Hospital, 30 Gascoigne Road, Kowloon, Hong Kong.
References
- Van der Heijde DM. Joint erosions and patients with early rheumatoid arthritis. Br J Rheumatol 1995;34 suppl 2:74-78.
- Fex E, Larsson BM, Nived K, et al. Effect of rheumatoid arthritis on work status and social and leisure time activities in patients followed 8 years from onset. J Rheumatol 1998; 25:44-50.
- Pincus T, O'Dell JR, Kremer JM. Combination therapy with multiple disease-modifying antirheumatic drugs in rheumatoid arthritis: a preventive strategy. Ann Intern Med 1999;131:768-774.
- Pincus T, Brooks RH, Callahan LF. Prediction of long-term mortality in patients with rheumatoid arthritis according to simple questionnaire and joint count measures. Ann Intern Med 1994;120:26-34.
- Pincus T, Sokka T, Wolfe F. Premature mortality in patients with rheumatoid arthritis: evolving concepts. Arthritis Rheum 2001;44:1234-1236.
- Wigley RD, Zhang NZ, Zeng QY, et al. Rheumatic diseases in China: ILAR-China study comparing the prevalence of rheumatic symptoms in northern and southern rural populations. J Rheumatol 1994;21(8):1484-1490.
- Boonen A, Chorus A, Miedema H, et al. Withdrawal from labour force due to work disability in patients with ankylosing spondylitis. Ann Rheum Dis 2001;60:1033-1039.
- Choy EHS, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med 2001;344(12):907-912.
- Maini.R, St Clair EW, Breedveld F, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet 1999;354:1932-1939.
- Lipsky PE, van der Heijde DM, St Clair EW, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group N Engl J Med 2000;343:1594-1602.
- Klareskog L, van der Heijde D, Jager JP, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet 2004;363:675-681.
- Bathon JM, Martin RW, Fleischmann RM, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med 2000;343:1586-1593.
- Weinblatt ME, Keystone EC, Furst DE, et al. Adalimumab, a fully human anti-tumor necrosis factor a monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate. Arthritis Rheum 2003;48(1):35-45.
- Braun J, Brandt J, Listing J, et al. Treatment of active ankylosing spondylitis with infliximab: a randomised controlled multicentre trial. Lancet 2002; 359:1187-1193.
- Braun J, Brandt J, Listing J, et al. Two year maintenance of efficacy and safety of infliximab in the treatment of ankylosing spondylitis. Ann Rheum Dis 2005;64:229-234.
- Van der Heijde D, Dijkmans B, Geusens P, et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheum 2005;52:582-591.
- Davis JC Jr, Van Der Heijde D, Braun J, et al. Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial. Arthritis Rheum 2003;48:3230-3236.
- Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumour necrosis factor a - neutralizing agent. N Engl J Med 2001;345(15):1098-1104.
