|
August 2002, Volume 24, No. 8
|
Update Article
|
||||
Enteral feeding and aspiration pneumonia in the frail patientS L Hui 許秀蘭,W H C Hu 胡興正,K W T Tsang 曾華德,C P Chung 鍾振邦,L W Chu 朱亮榮 HK Pract 2002;24:390-394 Summary Dysphagia and its consequence, aspiration pneumonia are common problems in the frail elderly, especially after strokes. Feeding tubes are inserted in dysphagic patients or patients who are otherwise unable to obtain adequate oral nutrition. Various types of feeding tubes are available and can be inserted either through the nose or percutaneously. This article reviews the different methods of feeding and the advantages and hazards associated with them. 摘要 吞嚥困難及其後遺症吸入性肺炎,在弱老及中風病人很常見。管飼飲食能幫助那些吞嚥困難或單靠口部進食不能得到足夠營養的病人。現有多種飼管可經鼻道或穿過腹部插入胃腸內以供餵飼。本文旨在分析不同的餵飼方法及其相關的好處和壞處。 Introduction Dysphagia and its major consequence, bronchial aspiration, are common events after neurological injuries. It has been estimated, by using clinical and videofluoroscopic assessment, that around 51% of patients may suffer from a swallowing disorder and 48% from aspiration after a first stroke.1 In another study, 67% of aspirations after a stroke were silent.2 Aspiration also correlated with subsequent development of pneumonia.3 More severe cases of dysphagia may even be associated with asphyxiation from food bolus inhalation. Aspiration and enteral feeding Oral aspiration Swallowing is a complicated process involving coordination of a number of muscle groups, resulting in the transfer of either liquid or food from the mouth into the stomach. Dysphagia and its main complication, aspiration pneumonia, may result from dysfunction of the oral, pharyngeal or oesophageal stages of swallowing. An American prospective study has suggested that in a long-term care setting, 56% of aspiration events led to radiographically proven cases of nosocomial pneumonia.4 Neurologic diseases, especially cerebrovascular accidents, are the commonest cause of dysfunction of the oral and pharyngeal stages of swallowing resulting in dysphagia. Dysphagic patients, fed orally, may aspirate during swallowing. They may aspirate food residue pooled in the pharynx.5 A videofluorographic study in 38 patients, suspected of having neurogenic dysphagia within four months of a stroke, found aspiration in 32%.6 In that study, delayed swallowing reflex was the commonest disorder (82%) and also the most frequent cause of actual aspiration of food. Other oropharyngeal motility problems in this cohort of patients included reduced pharyngeal peristalsis (58%), reduced lingual control (50%), reduced laryngeal adduction (5%) and cricopharyngeal dysfunction (5%). Most (76%) of patients suffered from more than one motility disorder and patients with a right-sided cerebrovascular accident showed a greater propensity to one component swallowing problems. Enteral feeding and pneumonia Non-oral feeding is a widely accepted means to feed patients with dysphagia at risk of aspiration. Non-oral feeding can be achieved via nasogastric tubes, thin bore nasoenteric tubes, or via surgically or endoscopically created gastrostomy and jejunostomy. The methods of feeding can be intermittent or continuous,7 and the formulae of feeds also differ from patient to patient.8 However, non-oral feeding does not prevent aspiration;9,10 there may be aspiration from oropharyngeal contents. Gastro-oesophageal reflux may also result in aspiration of feeds.11 In a prospective American study conducted over a 11-month period in a 527 bed nursing facility, 70 tube-fed patients were identified.12 Nasogastric tubes were used initially in 69 out of the 70 patients. Fifteen of them were subsequently converted to feeding via a gastrostomy tube. During the first two weeks, aspiration pneumonia developed in 43% of patients on nasogastric tube feeding and in 56% of patients with a gastrostomy. During the late period, corresponding figures were 44% among nasogastric tube patients and 56% among gastrostomy patients.12
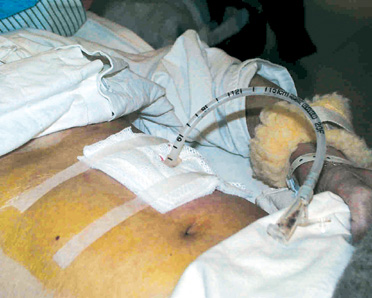
Pharyngeal aspiration and aspiration of refluxate It may be assumed that stopping oral intake of food may prevent aspiration. However feeding patients exclusively via gastric or jejunal tubes does not appear to be a completely effective strategy. In fact, one study showed similar rates of aspiration pneumonia preceding and after placement of a gastrostomy in neurologically disabled patients.13 There are other sources of aspiration in tube-fed patients; namely oropharyngeal secretions and refluxate from the stomach. In an important early study, Huxley et al studied pharyngeal aspiration by placing radioactive indium into the pharynx of 20 normal subjects and 10 with impaired consciousness. Forty-five percent of the normals and 70% of those with impaired consciousness aspirated from the pharynx.14 Apart from pharyngeal aspiration, gastro-oesophageal reflux is also common in critically ill patients. This may occur even in the absence of nasogastric tubes or enteral feeding. After ingestion of a meal, food accumulates in the fundus. Prevention of reflux involves two mechanisms - the basal tone of the lower oesophageal sphincter, and secondary oesophageal body peristalsis triggered by the presence of refluxate.15 Secondary peristalsis limits the extent and duration of reflux, pushing the refluxate bolus back into the stomach even when the first-line of defence, the lower oesophageal sphincter, is breached. Reflux may occur with decreased basal tone of the lower oesophageal sphincter, or more commonly, a transient relaxation of the sphincter.16 High gastric residual volumes may cause gastric distention and interrupt the integrity of the lower oesophageal sphincter.17 Coben et al examined lower oesophageal sphincter pressure before and after placement of gastrostomy tubes. Ten patients with a mean age of 81.7 were studied using standard water-perfusion manometry. Placement of gastrostomy tubes had no effect on basal lower oesophageal sphincter pressure. Rapid intragastric bolus infusion led to a reduction in the sphincter pressure. This was associated with free gastro-oesophageal reflux to the sternal notch as demonstrated by scintigraphy.18 In the same study, slow continuous feeding did not alter lower oesophageal sphincter pressure or cause reflux. Effects of bolus and continuous feeding It is commonly accepted that high gastric residual volumes, secondary to gastric distention, enhance regurgitation and may increase the risk for aspiration pneumonia. Fluids that commonly accumulate in the gastrointestinal tract of a tube-fed patient include the feeding formula, swallowed saliva (>0.8L/day), gastric secretion (1.5L/day) and small bowel secretion regurgitated into the stomach (2.7-3.7L/day).19 As the previously described study by Coben et al demonstrated,18 high residual volumes may similarly cause incompetence of the lower oesophageal sphincter and reflux of gastric contents. However, despite the theoretical advantages of continuous feeding, previous studies comparing intermittent with continuous feeding have mainly shown negative results. Ciocon et al randomly assigned 60 patients to either continuous or intermittent feeding.7 Diarrhoea was more common in patients fed intermittently and clogged tubes were more common in the continuously fed group. There was no significant difference in the incidence of pneumonia in the two groups, but the follow-up was for only 7 days. Another study of 34 neurosurgical patients fed by nasogastric tubes reported no significant difference between continuous and intermittent feeding with respect to various parameters, including aspiration.20 Effects of different feeding tubes It has been recommended in the United States that for tube feeding of more than 30 days, percutaneous gastrostomy or jejunostomy tubes would be preferred.21 Percutaneous gastrotomy was introduced over 20 years ago22 and has been widely used with good safety record. In the commonly employed "pull technique", the anterior abdominal wall is first punctured with a catheter. Positioning of the puncture site is confirmed with the aid of an endoscope placed inside the stomach. A guide wire is then passed through the catheter into the stomach. The intragastric portion of the guide wire is then pulled out of the mouth together with the endoscope. The end of the guide wire is then tied to a gastrostomy tube enabling the tube to be pulled back into the stomach and out through the previously created tract (Illustration 1).
Various studies have compared different forms of feeding. A 28-day study randomised 40 patients to either nasogastric tube or gastrostomy. 18 out of 19 nasogastric tube-fed patients were considered to have had treatment failure, compared to none in the gastrostomy group. Patients with a gastrostomy also had better weight gain and had more of the prescribed feeds.23 Another study randomised 33 patients into either intragastrical or post-pylorus feeding. 31.3% of gastric fed and 40% of post-pylorus fed patients developed radiographic aspiration pneumonia on follow-up;24 the difference was not statistically significant. In an intensive care setting, however, a study showed greater caloric intake, prealbumen rise and a trend for fewer pneumonia in jejunally fed patients, in comparison to gastrically fed controls.25 Tube size is probably not an important determinant of aspiration. A small-sized study comparing large and small bore feeding tubes in 25 patients have not shown any difference in the incidence of pneumonia.26 Another study examining reflux using gastro-oesophageal scintiscanning compared large and small bore nasogastric tubes in normal volunteers.27 No difference in the reflux index was apparent, suggesting that large bore nasogastric tubes do not cause more reflux than small bore tubes. Recognition and prevention of aspiration Clinical signs of aspiration in orally fed patients include coughing and choking during meals, or a wet hoarse voice after swallowing. Patients may also present with recurrent pneumonia and malnutrition. A high index of suspicion is needed for the diagnosis of aspiration in the elderly and frail patients. Apart from bedside diagnosis, more definitive investigations for aspiration include videofluoroscopic swallowing study (VFSS) and fibreoptic endoscopic evaluation of swallowing (FEES). Modification of food consistency, flavour or temperature as well as training by speech therapists may help the frail patient to swallow their meals better. In high risk patients or those with tendency to recurrent aspiration, tube feeding should be commenced. Conclusion Tube feeding is commonly employed in frail dysphagic elderly. Although the aim is to abolish recurrent pneumonia in these individuals, aspiration still occurs through reflux and aspiration of oropharyngeal contents. Percutaneous gastrostomy is preferable to nasogastric tubes for long-term non-oral feeding, providing greater patient comfort and also better nutrition. Jejunal feeding and continuous infusion feeding may have theoretical advantages over bolus gastric feeding but further research and controlled studies are needed to demonstrate actual benefits. For most patients, percutaneous gastrostomy with intermittent feeding will be sufficient. However, for those with recurrent aspiration pneumonia via percutaneous gastrostomy, continuous infusion feeding or conversion to jejunostomy should be considered as alternatives.
S L Hui, BNurs, RN
W H C Hu, MBBS(Lond), MRCP(UK), FHKCP, FHKAM(Med)
C P Chung, MBBS(HK), MRCP(UK)
L W Chu, MBBS(HK), FRCP(Edin), FHKCP, FHKAM(Med)
Associate Professor, Division of Respiratory Medicine, The University of Hong Kong. Correspondence to: Dr W H C Hu, University Department of Medicine, Queen Mary Hospital, Pokfulam Road, Hong Kong. References
|
|||||