|
September 2016, Volume 38, No. 3
|
Update Article
|
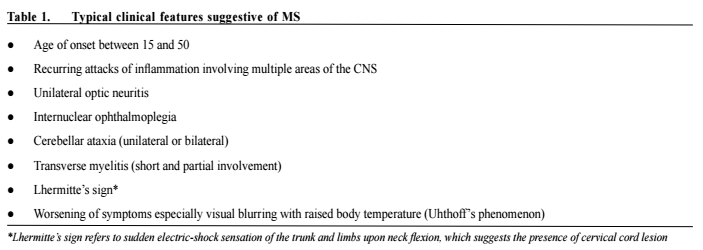
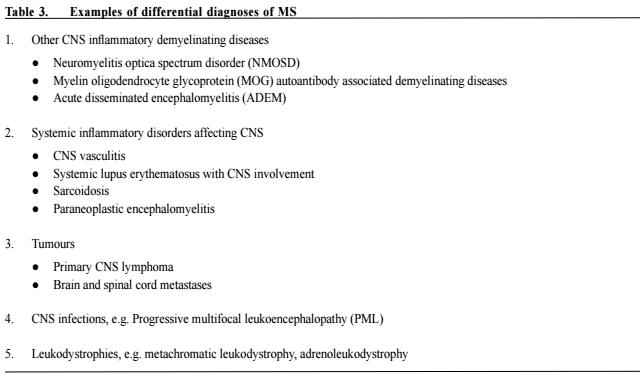
Multiple sclerosis - updates in managementChi-yan Lee 李志仁,Koon-ho Chan 陳灌豪 HK Pract 2016;38:83-92Summary Multiple sclerosis (MS) is an immune-mediated inflammatory demyelinating disease of the central nervous system (CNS). Majority of MS patients develop functional impairment as the disease progresses. In the old days, treatment of MS is mainly confined to treatment of acute relapses, symptomatic relief and rehabilitation. While there remains no curative treatment, numerous disease modifying therapies (DMTs) have emerged in the past decade and improved the long-term outcome and quality of life of patients with relapsing-remitting MS (RRMS). In this review, we discuss the management of MS with focus on the DMTs. 摘要 多發性硬化症(MS)是中樞神經系統(CNS)的壹種 免疫引導的炎性脫髓鞘疾病。隨著病情的發展,大部分 的MS患者都會出現功能性障礙。過去MS治療的主要目 標是治療病情急性復發,紓緩癥狀和康復理療。雖然目 前仍然沒有根治的方法,但是過去十年中,有很多改善 病情的藥物(DMTs)湧現,可以改善MS病人的長期療 效,提高多次緩解而又復發病患者(RRMS)的生活質 量。這次我們主要討論MS的治療方案中DMTs的作用。 lntroduction Multiple sclerosis is an immune-mediated inflammatory demyelinating disease of the central nervous system.1 The prevalence is highest among young female, with typical age of onset between 20 and 40 years. 80-85% of MS patients have an initial relapsing remitting course, characterised by acute relapses with new or recurrent neurologic deficits caused by inflammatory lesions at different sites. This is termed RRMS. Then, around 65% of RRMS patients enter the secondary progressive phase (SPMS), usually at around 40 years of age.2 In 20% of MS patients, the disease is progressive from onset (PPMS). Although the natural course of MS can be variable, majority of MS patients have significant physical and cognitive impairments 20 to 30 years after onset. The pathogenetic mechanism of MS is believed to be a complex multifactorial immune dysregulation, involving genetic susceptibility and environmental factors such as viral pathogens e.g. Epstein Barr virus, chemicals, smoking, obesity and vitamin D levels (sun exposure).3 The primary immune process involves migration of autoreactive lymphocytes across the blood–brain barrier in the CNS and the differentiation of memory T cells into pro-inflammatory T helper 1 (Th1) and Th17 lymphocytes.1,3,4 Other mediators of the inflammation, demyelination and axonal loss in MS include macrophages, microglial cells, CD8+ lymphocytes and memory B cells in the CNS.3,5 Prompt and effective treatment upon diagnosis is imperative to reduce morbidities of MS patients with active disease. Over the past decade, the treatment of MS has advanced significantly with multiple new disease-modifying therapies (DMTs) being tested and approved. While most MS patients are under neurology specialist care, doctors across different specialties including general practitioners also encounter MS patients on various treatments in their daily practice. Therefore, knowledge of the latest advances in MS diagnosis and management is essential. In this article, we discuss the clinical features and diagnosis of MS, and then review its management with focus on the conventional and newer DMTs. Diagnosis MS lesions can affect any part of the CNS and no clinical features are pathognomonic. Nonetheless, some sites, particularly optic nerves, cerebellum and spinal cord, are more frequently affected causing highly characteristic 84 The Hong Kong Practitioner VOLUME 38 September 2016 symptoms and signs. Table 1 listed the typical clinical features of MS and Table 2 listed the symptoms and signs of MS categorised by sites of involvement. At present, there is no specific diagnostic biomarkers available for MS. Diagnostic criteria include clinical and paraclinical assessments, emphasising the need to establish dissemination of lesions in space and time, and to exclude other diagnoses that can account for patients’ clinical presentation. Although clinical evidence can be sufficient to establish the diagnosis of MS, magnetic resonance imaging (MRI) of the CNS is an increasingly important assessment tool. It can support or even replace some clinical criteria. Look for atypical features suggestive of alternative diagnoses. Other investigations including cerebrospinal fluid (CSF) analysis and visual evoked potentials facilitate the diagnosis but are not confirmatory. They are particularly useful in ambiguous situations. The 2010 revisions to the McDonald Criteria of the International Panel on Diagnosis of MS6 highlighted the importance of MRI and simplified the criteria. Replacing the Barkhof criteria which require more lesions, dissemination in space can now be demonstrated with at least 1 T2 lesion in at least 2 of 4 locations considered characteristic for MS (juxtacortical, periventricular, infratentorial, and spinal cord), with lesions within the symptomatic region excluded in case of brainstem or spinal cord syndromes. This allows for safe and early diagnosis, aiming at a timely initiation of DMTs. However, one must be aware that there are other diseases mimicking MS with similar clinical and radiological features, which might also fulfill the diagnostic criteria. Therefore, clinical judgment should be exercised when applying the diagnostic criteria. Excluding alternative diagnosis is crucial and specific investigations, e.g. serum aquaporin-4 autoantibody testing, CSF for John Cunningham virus (JCV) detection, or brain biopsy should be performed in appropriate settings. Table 3 listed some important examples of differential diagnoses of MS. Management The management of MS can be divided into 1) treatment of acute relapses, 2) modifications of disease course by DMTs to prevent relapse and long-term disability and 3) relief of neurological symptoms and deficits. In relapses with acute symptomatic worsening, high-dose methylprednisolone (0.5–1g per day), usually given intravenously as a 3- to 5-day course, remains the standard treatment.7 Persistent deficits with suboptimal response to corticosteroid treatment can be reduced with plasmapheresis given up to 1 month after onset.8,9Before the more widespread use of DMTs specific for MS, azathioprine, a traditional immunosuppressant, had been used to treat MS with variable effects. Azathioprine is a purine antimetabolite that inhibits RNA and DNA synthesis, thereby suppresses T and B lymphocyte function, and possibly dendritic differentiation.10 A metaanalysis of 7 randomised controlled trials (RCTs) showed MS patients treated with azathioprine had less relapses (relative risk reduction of about 20%) but only marginal benefits in disability status for 2 to 3 years of treatment.11 A more recent Cochrane systematic review revealed similar findings and commented azathioprine as a fair alternative to beta-interferon (β-IFN) in patients with frequent relapses requiring steroids.12 Common side effects include gastrointestinal symptoms including nausea and abdominal discomfort. Other side effects include mild bone marrow suppression and hepatotoxicity which usually improve with dose reduction. Pancreatitis, severe leucopenia or pancytopenia, idiosyncrastic reaction manifesting as prominent nausea, vomiting and abdominal discomfort and severe liver function derangement are infrequent but require treatment withdrawal. A possible increased risk in malignancy is present especially with longer treatment duration and higher cumulative dose. Longterm use (> 10 years) or cumulative doses > 600g should be avoided.12 With safer and more effective DMTs coming into market, use of azathioprine in MS has been largely dismissed nowadays.
The first approved DMT for MS, interferon beta-1b, was introduced in 1993. Since then, conventional injectable DMTs namely β-IFN and glatiramer acetate (GA), have been the mainstays of MS treatment. In recent years, several other DMTs including oral therapies and highly active monoclonal antibodies became available. Although effective treatment to halt progressive phase of MS is lacking, RRMS should now be considered treatable with the recent advances. At the same time, treatment of MS is becoming more complex and decision to choose among various DMTs depends on characteristics of individual patient, adverse effect profiles and tolerability, local availability and costeffectiveness. Below, we review the present and emerging DMTs of RRMS. Disease modifying therapies Beta-interferon (β-IFN)β-IFN 1a and 1b are first-line DMTs for RRMS. Its mechanisms of action include: 1) anti-inflammatory effects via inhibition of T cell proliferation, modulation of T cell functions (reducing production of Th1 proinflammatory cytokines and shifting the immune response toward a Th2 profile) and modulation of B-cell functions, 2) reducing expression of matrix metalloproteinases, and 3) reducing migration of inflammatory cells from peripheral blood to the CNS via reversal of blood-brain barrier disruption.13 Several phase 3 clinical trials have consistently demonstrated efficacy of β-IFN in reducing relapse frequency (by ~30%- 35%)14-16 and disease activity shown on MRI17,18, and slowing disability progression over the short duration of the clinical trials. Nonetheless, long-term benefits on decreasing disability accumulation or delaying onset of secondary progression are uncertain. β-IFNs are given subcutaneously or intramuscularly, from alternate-daily to weekly dosing, depending on different preparations. In general, β-IFN therapy is well tolerated. Common side effects include flulike symptoms (e.g. fever, malaise, myalgia, headache, chills and rigor), injection site reactions, elevated transaminases and depression. Flu-like symptoms are common and may be prominent during initiation of therapy but usually subside or lessen with continued therapy. Elevation of transaminases are not uncommonly observed with β-IFN therapy which is reversible and will not cause persistent hepatic injury. Persistently elevated transaminases of more than 3 times the upper limit of normal is an infrequent cause of withdrawal of β-IFN therapy. A subgroup of patients treated with β-IFN will develop neutralising antibodies, which are associated with reduced efficacy of these agents.19 While testing for these antibodies can be performed if a patient has evidence of clinical or MRI disease activity despite good compliance to β-IFN therapy, replacement with another class of DMT should be considered regardless of the antibody status. Use of β-IFN can also be considered as treatment for SPMS with ongoing relapses and clinically isolated syndrome (CIS) with high risk of progression to definite MS. Glatiramer Acetate (GA)GA is a pool of synthetic peptides (average length 40- 100 residues) with amino acid sequences similar to myelin basic protein. It has widespread effects on the innate and adaptive immune systems leading to anti-inflammatory action via deviation to Th2 response with development of glatiramer acetate reactive Th2 CD4+ T cells. These cells accumulate in the CNS and promote bystander suppression by releasing anti-inflammatory cytokines. It also induces regulatory T cells and inhibits myelin reactive T cells. Treatment requires subcutaneous injection. GA reduces relapse rate by approximately 30% in RRMS20 and confers improvement in MRI measures of disease activity.21 The efficacy in RRMS and ability to delay progression of CIS to definite MS are comparable to β-IFN. Similar to β-IFN, GA has established long-term safety as there are no reports of increased risk of cancer or infections with prolonged therapy. GA is usually well tolerated. The most common side effects are injection site reactions (pain, erythema, swelling and pruritus) which develop in 65% of patients. About 15% of patients develop a transient self-limited reaction immediately after injection manifested as facial flushing, chest tightness, palpitation, anxiety and dyspnea. Other reported side effects include lymphadenopathy and lipoatrophy. NatalizumabNatalizumab is a humanised monoclonal antibody acting on the α4-integrin (VLA-4) on leucocytes (mainly lymphocytes) which binds to vascular cell adhesion molecules (VCAM-1) on endothelial cells. Binding of VLA-4 on leucocytes to VCAM-1 on endothelial cells allow leucocytes from peripheral blood to cross the bloodbrain- barrier (BBB) and enter into the CNS. Hence, the mechanism of action of natalizumab is largely via blocking α4-integrin, preventing adherence of activated leucocytes to inflamed endothelium and hence inhibiting migration of activated leucocytes across the BBB into the CNS. Monthly intravenous infusions of natalizumab were shown to reduce annualised relapse rate (ARR) by 68% over 2 years, disability progression by 42% and new gadoliniumenhancing lesions on MRI by 92%.22 In another phase 3 study, combination treatment with natalizumab and β-IFN 1a was more effective than β-IFN 1a monotherapy.23 Natalizumab is recommended for RRMS patients with active disease despite use of standard first-line DMTs, e.g β-IFN or GA, or those with an early aggressive disease course (at least 2 relapses per year) who would likely develop disability early. Like β-IFN therapy, 6% of patients develop persistent neutralising antibodies against natalizumab which may reduce drug efficacy.24
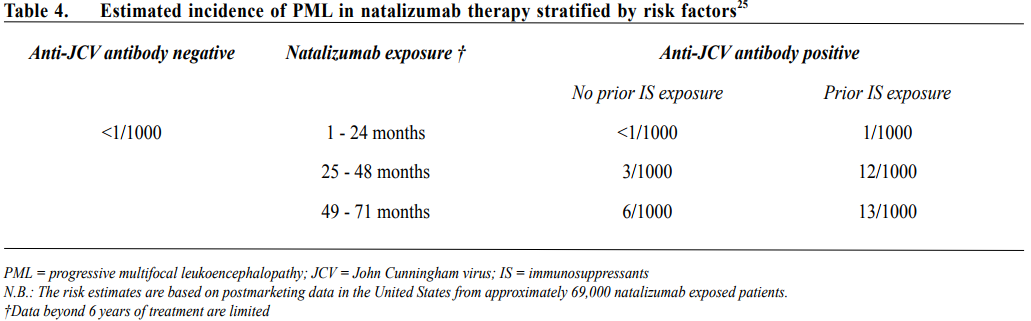
The main safety concern of natalizumab therapy is a non-negligible risk of progressive multifocal leukoencephalopathy (PML). PML is a life threatening opportunistic infection of oligodendrocytes due to JCV reactivation. Risk factors for development of PML with natalizumab therapy include seropositivity for anti-JCV antibody, previous exposure to immunosuppressive therapy and longer duration of natalizumab treatment. Table 4 showed the estimated incidence of PML in natalizumab therapy stratified by risk factors.25 Throughout period of natalizumab therapy, especially beyond 24 months of treatment, frequent clinical and MRI monitoring (every 3-6 months), and interval anti-JCV antibody testing in patients with initial negative results are mandatory to look for early evidence of PML and decide on total duration of treatment. PML associated with natalizumab therapy commonly presents with motor symptoms of hemiparesis and ataxia, visual field defects and cognitive changes, may be misdiagnosed as relapse of MS. MRI abnormalities detected in the asymptomatic stage are particularly useful as prognosis is better with early detection and withdrawal of natalizumab therapy. Polymerase chain reaction (PCR) for JCV DNA in CSF helps to confirm a diagnosis of PML though in some patients, brain biopsy is needed when CSF is repeatedly negative for JCV DNA. Once PML associated with natalizumab therapy is diagnosed, natalizumab should be stopped and plasmapheresis performed to promote rapid drug removal. There is no known effective treatment and the mortality rate is 20-25% with significant neurological disabilities in the majority of survivors.26 Patients may have neurological deterioration after cessation of natalizumab therapy with enlargement and oedema of brain lesions on MRI. This is believed to be due to immune reconstitution inflammatory syndrome (IRIS) arising from increased entry of leucocytes into the CNS upon withdrawal of natalizumab. Intravenous immunoglobulins (IVIg) may help to attenuate the inflammatory response in IRIS. FingolimodFingolimod is the first approved oral DMT for RRMS. It is a sphingosine-1-phosphate (S1P) analogue and acts via S1P receptor modulation. Fingolimod binds to S1P receptors on surface of lymphocytes leading to internalisation and hence reduction of S1P receptors on surface of lymphocytes. S1P receptors are needed for egression of lymphocytes from lymph nodes. Fingolimod impedes the egression of lymphocytes from lymph nodes, thus inhibits the migration of T cells into the circulation and target organs including the CNS. Two phase 3 trials have confirmed its superior effectiveness over placebo and weekly β-IFN 1a in reduction of ARR and disease activity.27,28 In Hong Kong, fingolimod is indicated as second line DMT for RRMS. Although fingolimod is in general well tolerated, several safety concerns are noteworthy, including: 1) firstdose bradycardia and atrioventricular conduction block, 2) varicella zoster (VZV) infections, 3) lymphopenia, 4) macular edema and 5) liver function derangements. Therefore, pretreatment workup and continuous monitoring are important for fingolimod therapy, especially in patients with pre-existing cardiac, ocular or hepatic diseases. Patients who are seronegative for anti-VZV antibody should receive VZV vaccine before initiation of fingolimod therapy. In addition, in August 2015, the United States Food and Drug Administration (FDA) announced that a case of definite PML and a case of probable PML were reported in patients taking fingolimod for MS. Both patients had not been previously treated with any immunosuppressant drug. Overall, the risk of PML with fingolimod is believed to be less than natalizumab, but long-term safety data is lacking. Besides, fingolimod may be teratogenic and strict contraceptive measures are needed for patients on therapy. TeriflunomideTeriflunomide, a metabolite of leflunomide, is a reversible inhibitor of the mitochondrial enzyme dihydroorotate dehydrogenase. The enzyme is required for de novo pyrimidine synthesis in proliferating lymphocytes. Teriflunomide inhibits proliferation of stimulated T and B lymphocytes in the periphery which are considered to be responsible for the neuroinflammation of MS and diminishes the number of activated T and B lymphocytes available for migration into the CNS. Teriflunomide therapy has no effect on basic homeostatic cell functions of resting lymphocytes or normal immune surveillance. In two phase 3 clinical trials, teriflunomide oral therapy given 14mg once daily reduced ARR, disability progression and improved MRI outcomes significantly compared to placebo.29,30 Its effect was shown to be comparable to subcutaneous preparation of β-IFN 1a, but no superiority or statistically significant clinical benefit as add-on treatment to β-IFN 1a was found. Teriflunomide is approved as first line DMT for RRMS. Common adverse events associated with teriflunomide include nausea, diarrhoea, hair thinning and elevated parenchymal liver enzymes (observed in ~14%). Monthly liver function monitoring for the first 6 months is recommended. Live attenuated vaccines are contraindicated in patients treated with teriflunomide. Risk of teratogenicity is a serious concern for women and possibly for men as well. Teriflunomide has a long half-life of about 19 days and may take months to be completely eliminated from the body. The elimination can be accelerated using cholestyramine or activated charcoal given as an 11-days course, e.g. before conception or switching to another DMT with immunosuppressive effects. Dimethyl Fumarate (DMF)DMF is a fumaric acid ester and BG-12 is a formulation of it produced as an enteric-coated microtablet. BG-12 is given as a twice or thrice daily oral therapy. The exact mechanisms of the immunomodulatory or neuroprotective effect of DMF are uncertain but it is thought to act through activation of the Nrf-2 pathway. BG-12 was shown to reduce ARR, disability progression and disease activity shown on MRI of RRMS patients in two RCTs31,32, but there was no conclusive evidence for superiority over GA.32 BG-12 is approved as first line DMT in RRMS. BG-12 was not shown to associate with significant infections or malignancy, but might cause lymphopenia or increase in minor infections. The most frequent side effects include flushing, diarrhoea, nausea and abdominal pain. Of note is that cases of PML have been reported with use of Fumaderm, an oral drug used in the treatment of psoriasis and is composed of a mixture of fumaric acid esters including DMF. Long-term safety data in MS are not available yet. Like teriflunomide, administration of live attenuated vaccines is not recommended during DMF therapy. Animal studies showed teratogenicity, and consequently a washout period of one month is recommended before conception. AlemtuzumabAlemtuzumab is a humanised monoclonal antibody targeting CD52, a 12 amino acid glycosylated glycosylphosphatidylinositol-linked protein expressed on the surface of lymphocytes, monocytes, macrophages, eosinophils and NK cells. It causes rapid and profound depletion of both T and B lymphocytes via antibodydependent cell-mediated cytotoxicity. As CD52 is not expressed on haematopoietic precursors, beneficial immune reconstitution follows alemtuzumab therapy. Within weeks after treatment, the lymphocytes begin to recover at different rates with CD4+ T lymphocytes being the slowest. B cells usually return to normal within 3 to 6 months but T cells take approximately 12 months to recover but never return to baseline.33 This depletion and repopulation process leads to sustained changes in T cell immunity. In treatment of RRMS, alemtuzumab is given as a daily intravenous infusion for 5 days at initiation and 3 days at 12 months. Further infusion is usually not required. Three phase 3 randomised controlled trials34-36 have confirmed the treatment efficacy of alemtuzumab in RRMS over β-IFN 1a. There was significant reduction in ARR, disability progression and disease activities shown on MRI. Importantly, sustained reduction in disability, defined as a ≥ 1 point decrease on the expanded disability status scale (EDSS) sustained for 6 consecutive months for patients with a baseline EDSS ≥2, was more likely among patients treated with alemtuzumab than patients treated with β-IFN 1a.37 The treatment effects could last up to 5 years without further treatment course. Alemtuzumab is approved for active MS and used as second or third line DMT in general. Infusion reactions including headache, fever and skin rash are very common (90%) with alemtuzumab therapy but are rarely serious. These reactions can be ameliorated with slower infusion rate and premedications (steroids, paracetamol and antihistamines). Development of secondary autoimmune disorders is a major concern of alemtuzumab therapy in MS, in which up to 30% of patients develop autoimmune thyroid disorders, followed by immune thrombocytopenic purpura (3%) and Goodpasture syndrome (0.5%).38 Other rarely reported autoimmune disorders include autoimmune haemolytic anaemia, autoimmune pancytopenia, autoimmune neutropenia, autoimmune hepatitis, anti-phospholipid syndrome, alopecia and vitiligo. Cases of autoimmune neutropenia and dermatological disorders (e.g. vitiligo, bullous skin rash) were also reported. The cumulative risk for secondary autoimmune diseases is 22.2%, which most frequently develop between 12 and 18 months following the first dose and can be evident for up to 5 years.39 Regular monitoring of blood counts, renal and thyroid functions are essential for alemtuzumab therapy. Alemtuzumab therapy also increases risks of infections, most commonly involving the upper respiratory tract, urinary tract and oral herpes, but these are usually manageable and rarely fatal. PML has been observed in patients treated with alemtuzumab for lymphoproliferative disorders, but so far has not been reported in MS patients. MitoxantroneMitoxantrone is an antineoplastic anthracenedione derivative that inhibits topoisomerase II. It was approved as a single agent for the treatment of aggressive RRMS, SPMS and PPMS. In a Cochrane meta-analysis40 that includes a total of 221 subjects with follow-up up to 2 years, mitoxantrone was shown to reduce ARR, disease activity shown on MRI, disability progression at 24 months and increase the proportion of relapse-free patients at 1 and 2 years.
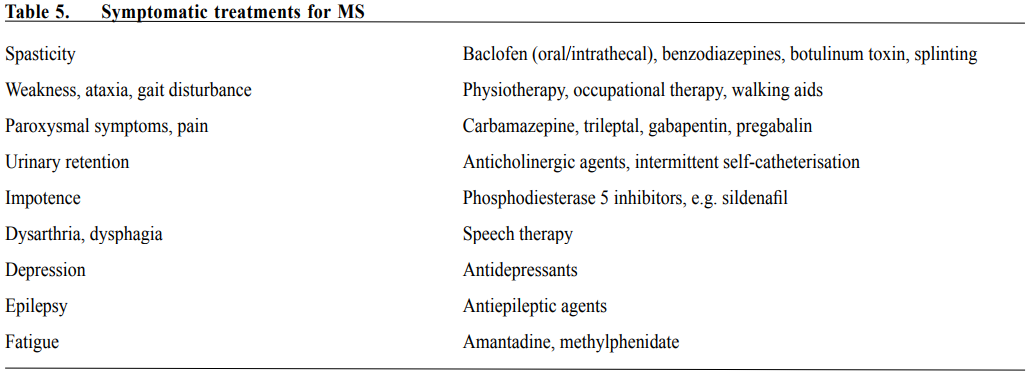
The major concern for the use of mitoxantrone in MS is the long-term severe adverse effects, namely opportunistic infections due to myelosuppression, cardiotoxicity including reduction of left ventricular ejection fraction and congestive heart failure, therapy-related acute leukaemia, reduced fertility with persistent amenorrhoea and teratogenicity. Furthermore, previous exposure to mitoxantrone increases the risk of PML in MS patients requiring natalizumab therapy and possibly other DMTs. With increasing recognition of the unfavourable safety profile and the introduction of newer efficacious DMTs, mitoxantrone is rarely used to treat MS in recent years, but it remains an option for MS patients with aggressive disease refractory to other DMTs. Local experiences in the use of DMTs In Hong Kong, β-IFN is the standard first-line DMT for RRMS patients and have been available for patients for more than 15 years. However, β-IFN therapy in local MS patients has been limited for more than 10 years since availability due to discomfort and inconvenience associated with regular injection and high cost. Since 2012, β-IFN is provided by Hospital Authority (HA) as a special drug (paid by HA) and becomes increasingly used as first-line DMT for local RRMS patients. GA is seldom used due to its need of daily injection and is not available from HA. RRMS patients who do not have satisfactory response to β-IFN (clinical relapses and/or MRI evidence of active disease with adequate trial of β-IFN therapy for at least 12 months) will be considered for second-line DMTs. Fingolimod is a commonly used second-line DMT in our population due to convenience of oral intake and availability of financial support from Samaritan Fund for its usage in patients with financial restrain. However, some local RRMS and relapsing progressive patients develop severe prolonged lymphopenia with fingolimod requiring withdrawal of therapy. Patients who have aggressive RRMS characterised by frequent relapses (2 or more relapses in a year) especially those with severely disabiling relapses may benefit from natalizumab for 6-12 months to stabilise the disease followed by de-escalation to β-IFN or fingolimod with lower risk of PML compared to prolonged natalizumab therapy. In patients with severe active MS who do not respond to or cannot tolerate β-IFN, fingolimod and/or natalizumab, alemtuzumab can be considered. We do not have experience with the use of dimethyl fumarate and teriflunomide. Symptomatic treatment Apart from treatment of acute relapses and use of DMTs, relief of symptoms is also important in managing MS patients. Table 5 shows some commonly used symptomatic treatments. Non-pharmacological treatments Non-pharmacological treatments also play important role in the management of MS patients. Diverse neurological disabilities may develop in the patients, physiotherapy and occupational therapies especially after recent relapse are beneficial to hasten recovery and optimise neurological functions for activities of daily living and working capacity. On the long-term, regular physical exercise may be beneficial for neurological functions and protect against depression. Cognitive functions should be formally assessed by clinical psychologist and those with cognitive impairment may benefit from cognitive rehabilitation. Patients with sphincter dysfunction should be assessed by urologist and may benefit from pelvic floor exercise, some with urinary retention may require intermittent urinary catheterisation or long-term in-dwelling catheter. MS is a chronic debilitating disease and patients are at increased risk of depression. Clinicians caring for them should be alert to symptoms suggestive of depression. Prompt referral for psychiatric assessment is important. Other psychiatric symptoms such as anxiety, aggression and psychosis as a result of structural cerebral injury have been reported in MS infrequently. Conclusion Diagnosis of MS has improved with the increased clinical experience and advancement of MRI. More RRMS patients will receive early DMT, aiming at reduced relapse frequency, better long-term outcome with less disabilities and better quality of life. Options of DMTs have grown substantially in the past decade, and more are expected to be approved in the near future. Although the conventional injectable DMTs remain the standard first-line treatment in RRMS, the advent of efficacious oral DMTs have improved patients’ quality of life and compliance. Furthermore, more potent DMTs are now available for RRMS patients with early aggressive disease or suboptimal responses to the firstline DMTs. On the other hand, these more potent DMTs are associated with potentially serious adverse effects and safety issues. Careful evaluation of potential benefits and risks of the newer DMTs must be performed on an individual basis, and thorough understanding of these newer agents is essential. As diverse neurological disabilities can develop in MS patients, a multi-disciplinary approach of management is the ideal.
Chi-yan Lee, MBBS, FHKCP, FHKAM (Medicine)
Resident Specialist Department of Medicine, Queen Mary Hospital Koon-ho Chan, MD, PhD, FHKCP, FHKAM (Medicine) Clinical Associate Professor Department of Medicine, The University of Hong Kong Correspondence to: Dr Koon-ho Chan, Room 405B, 4/F, Professorial Block, Department of Medicine, Queen Mary Hospital, 102 Pokfulam Road, Hong Kong SAR, China. E-mail: koonho@hkucc.hku.hk
References
|
|